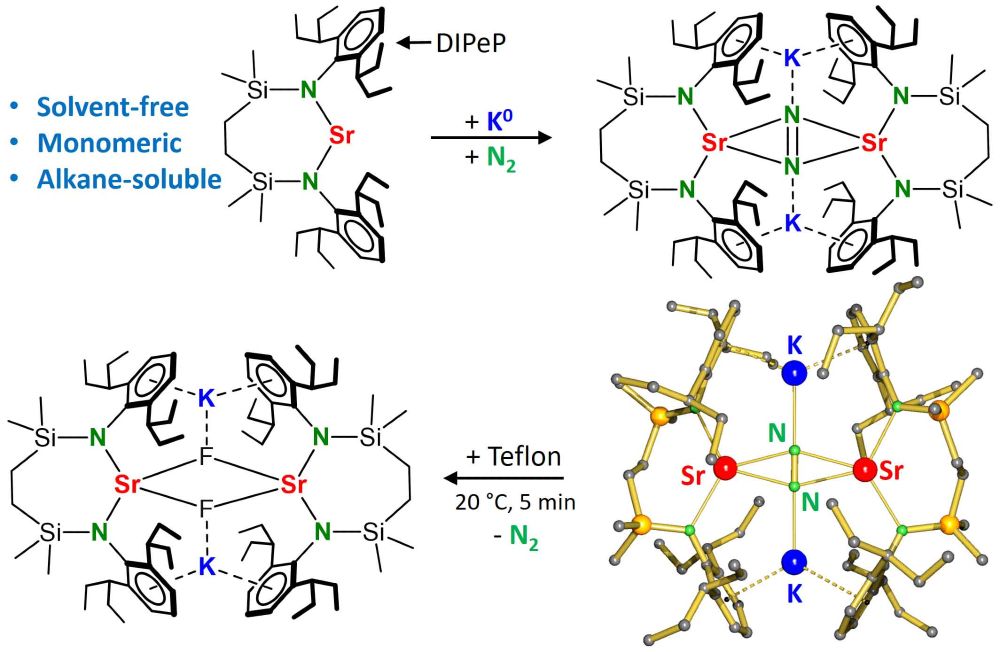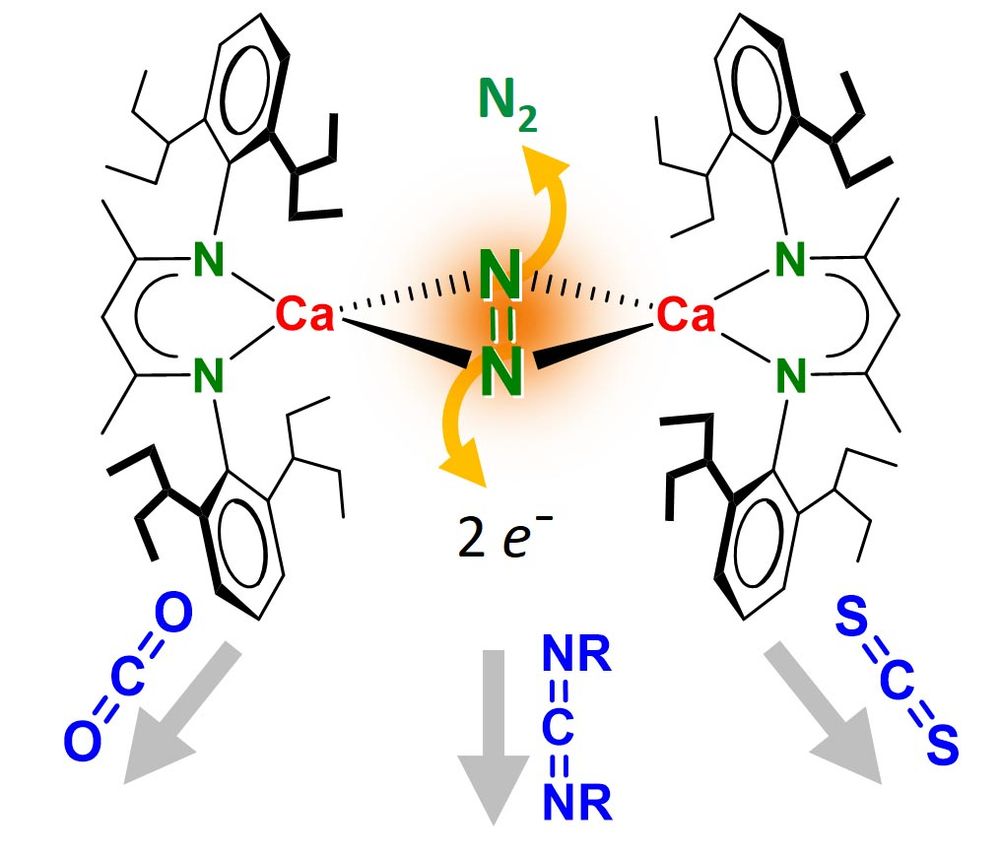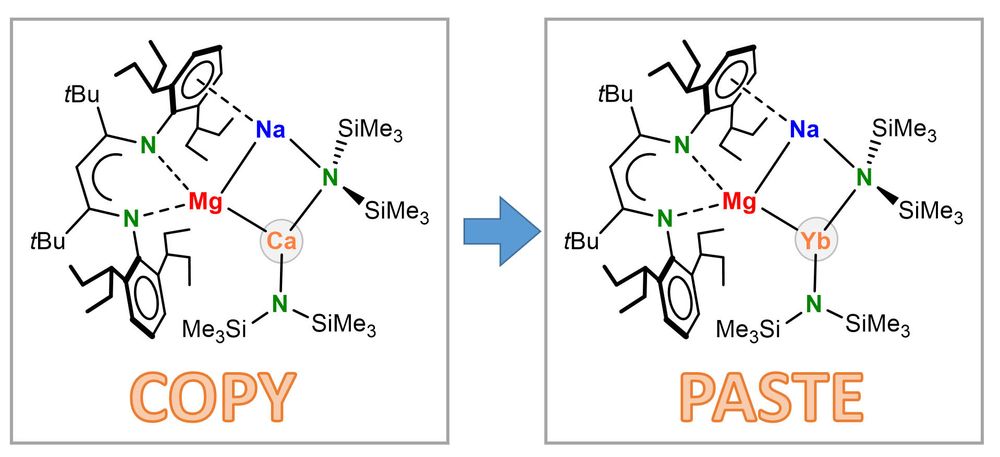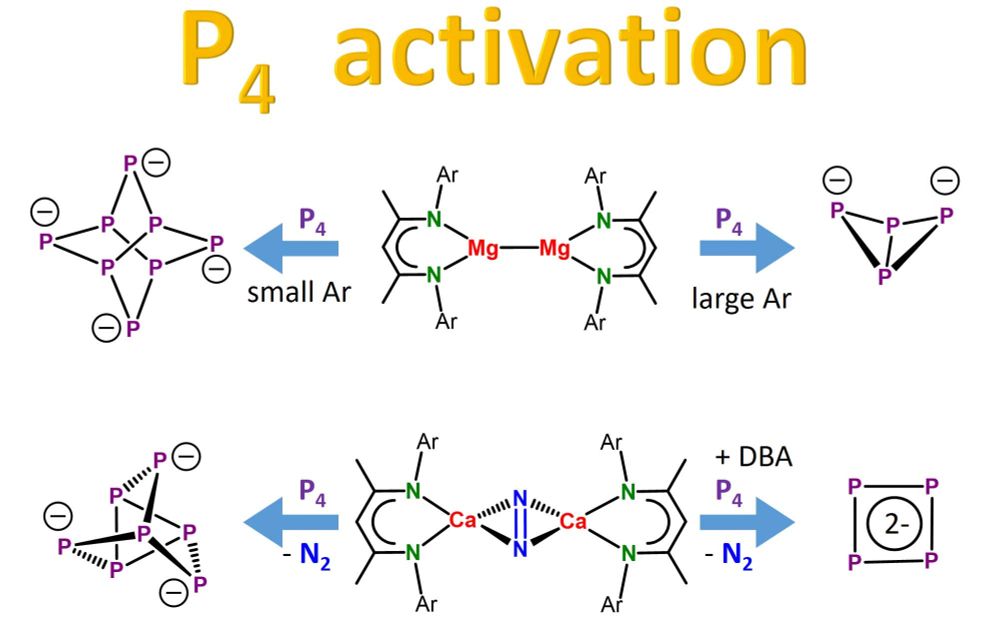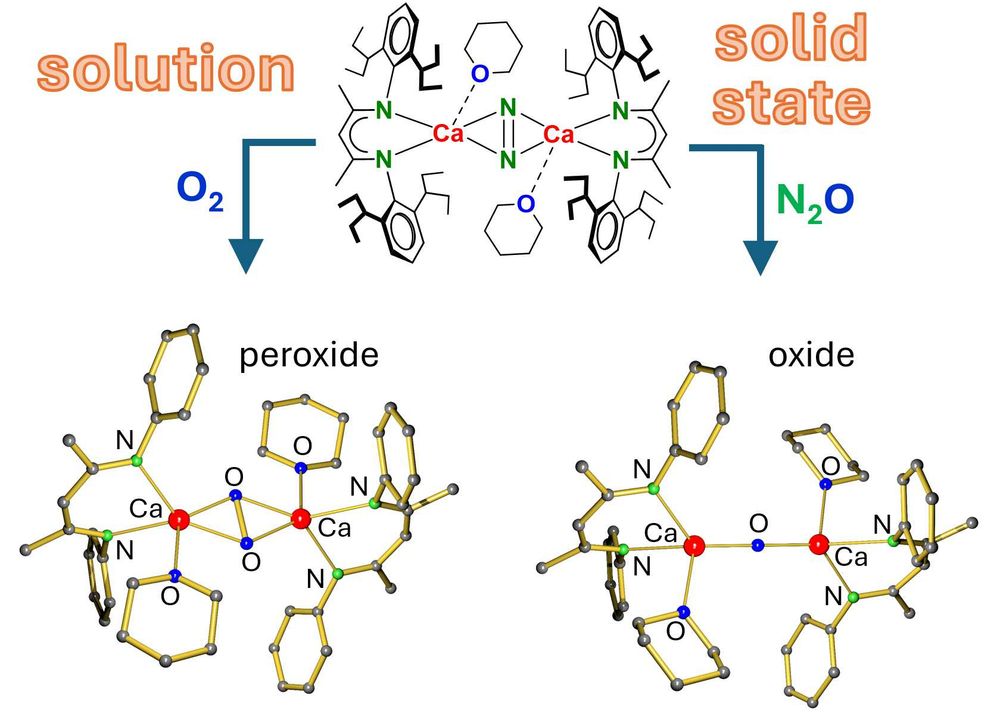
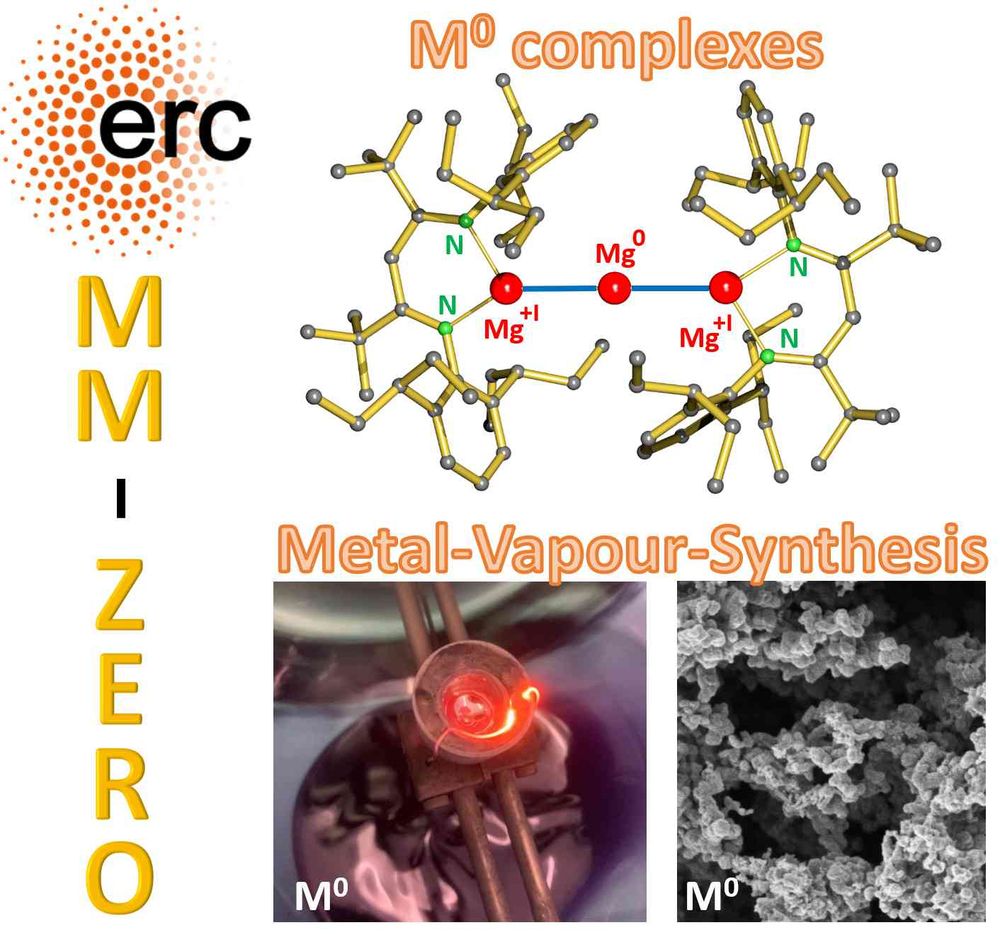
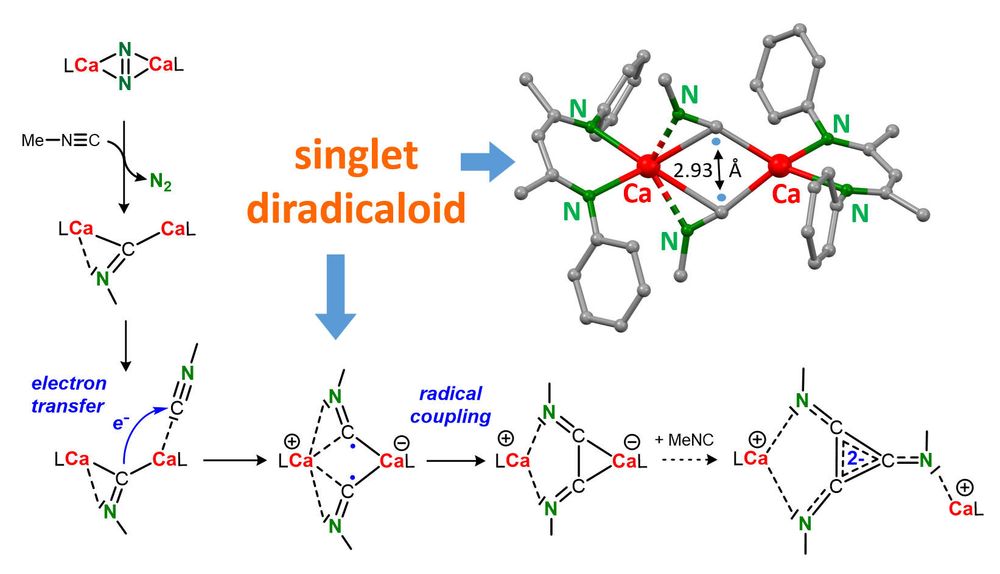
Hydrocarbon-soluble Mg(0) reduces Si all the way to Si(4-). Unfortunately not stable and decomposing to the HSi(3-) anion. However, Sn(4-) is stable and functions as 4-fold Nu or 8e reducing agent. Preprint: shorturl.at/4mH0O
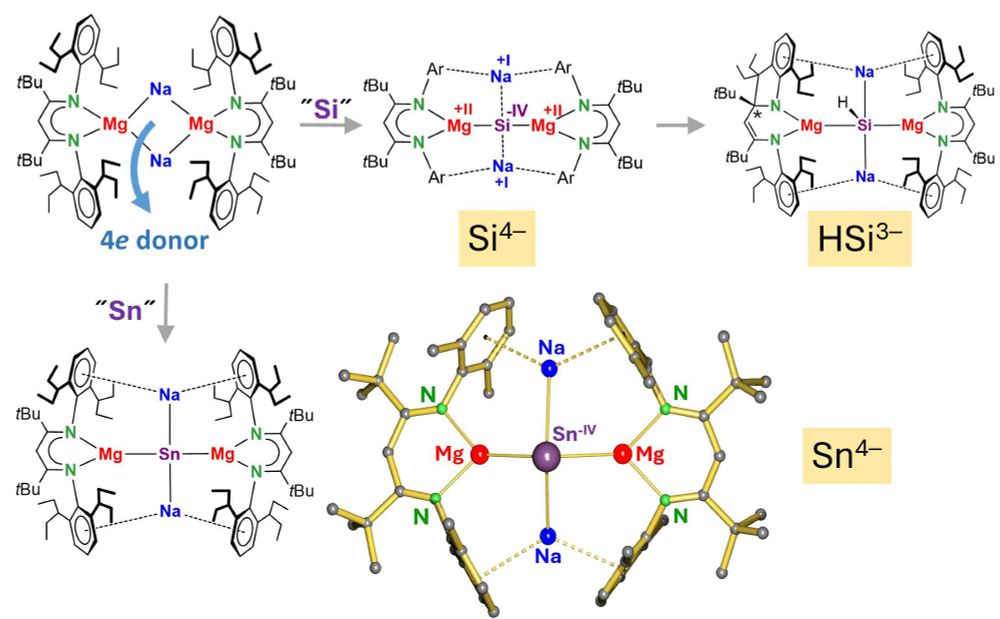
Hydrocarbon-soluble Mg(0) reduces Si all the way to Si(4-). Unfortunately not stable and decomposing to the HSi(3-) anion. However, Sn(4-) is stable and functions as 4-fold Nu or 8e reducing agent. Preprint: shorturl.at/4mH0O