
Very pleased this work is out in JACS. Congrats Dr. Almquist!

Very pleased this work is out in JACS. Congrats Dr. Almquist!




Heavyweight Champion: Caesium Diorganophosphides Outperform Lighter Congeners in Catalytic Hydrophosphination Angewandte Chemie International Edition - Wiley Online Library onlinelibrary.wiley.com/doi/10.1002/...
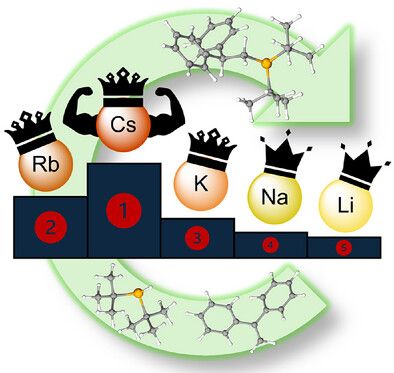



pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...
So proud to congratulate first author Leon Gomm for his first paper in JACS. Thanks to Hui Zhu and Stefan Grimme for the ever helpful DFT calculations. @unibonn.bsky.social
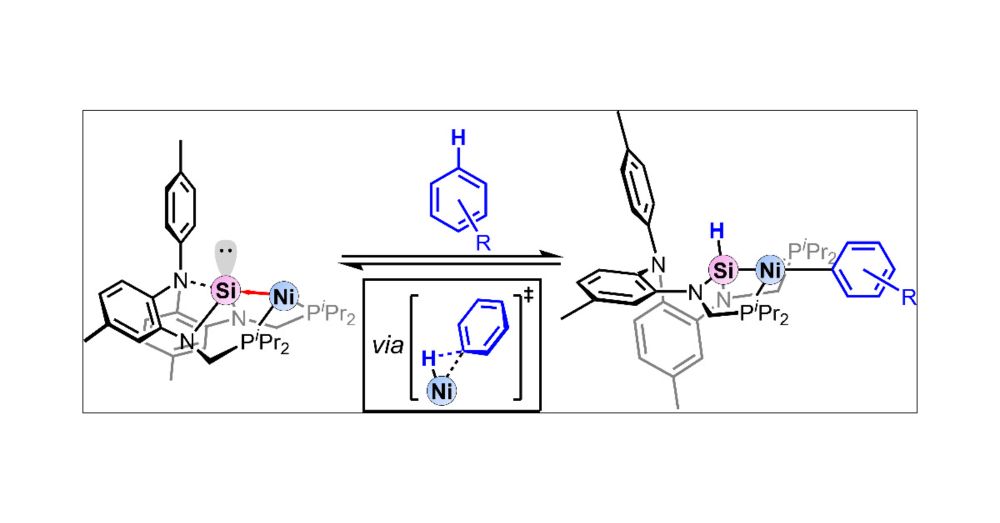
pubs.acs.org/doi/10.1021/...
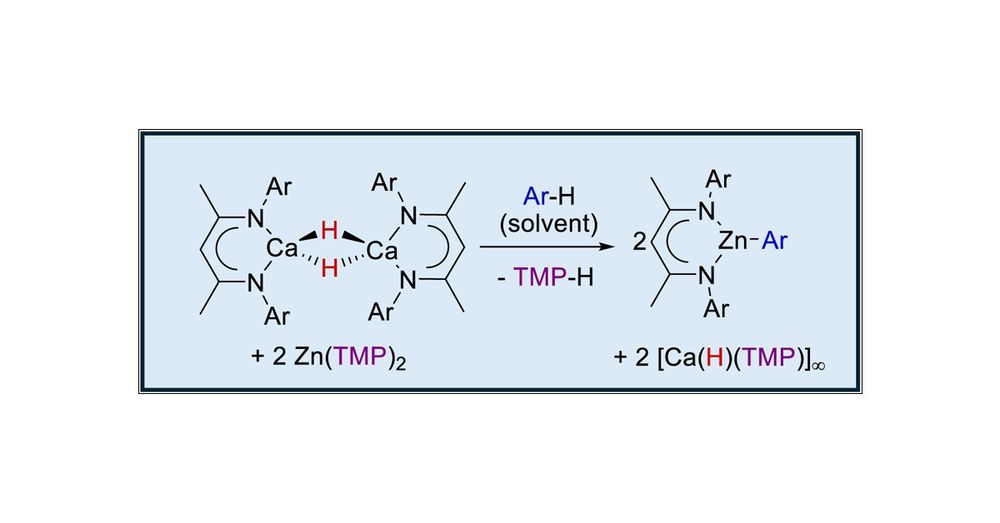
pubs.acs.org/doi/10.1021/...

pubs.rsc.org/en/content/a...
📍 @ox.ac.uk, @vicuniwgtn.bsky.social
#Chemsky 🧪
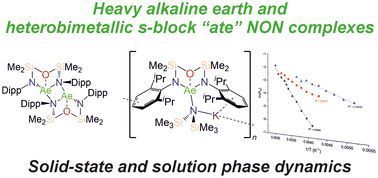
pubs.rsc.org/en/content/a...
📍 @ox.ac.uk, @vicuniwgtn.bsky.social
#Chemsky 🧪

Published as part of Organometallics special issue “Organometallic Chemistry Beyond the Transition Metals:
Fundamentals and Applications of the P-Block”
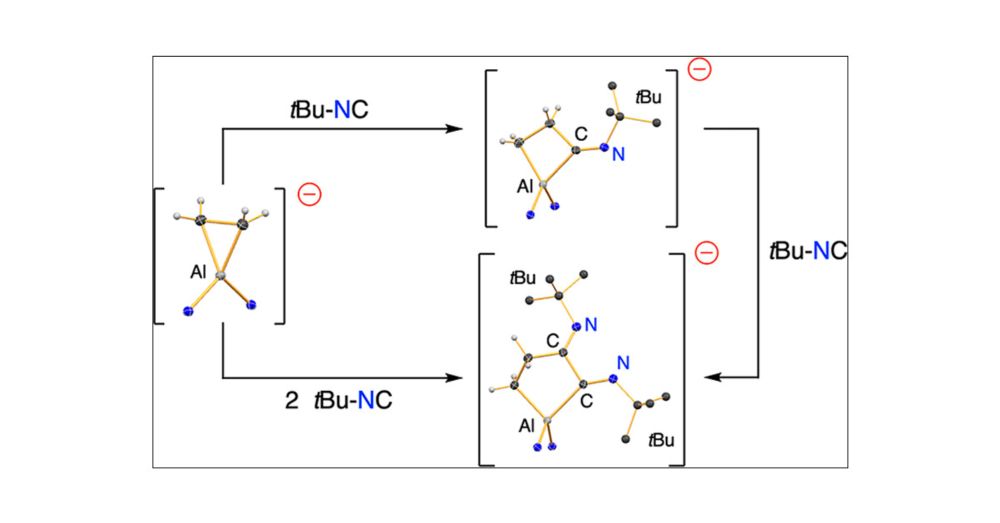
Published as part of Organometallics special issue “Organometallic Chemistry Beyond the Transition Metals:
Fundamentals and Applications of the P-Block”



pubs.acs.org/doi/10.1021/...
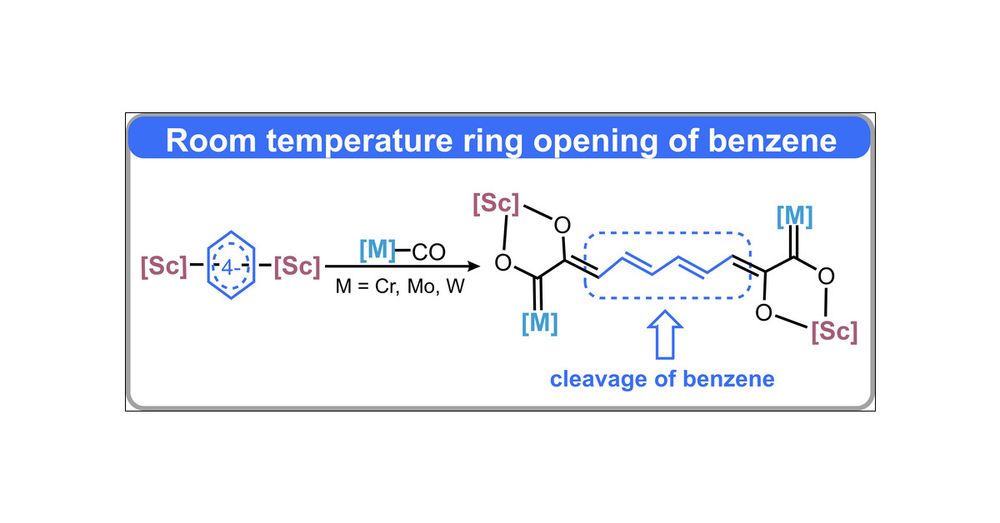
pubs.acs.org/doi/10.1021/...
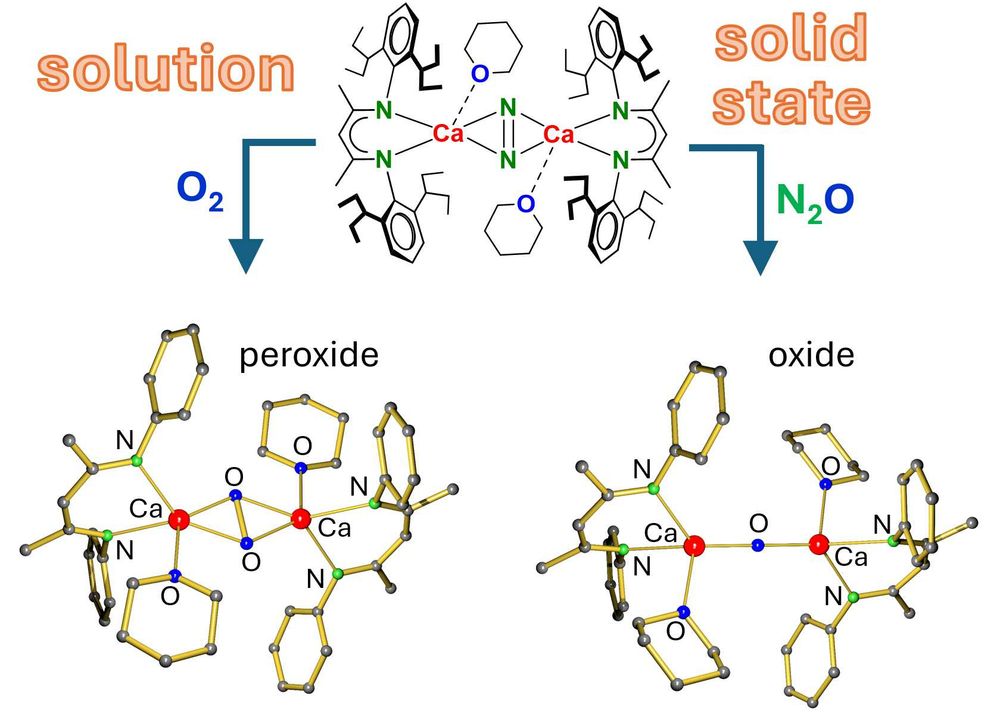

Mehr Infos: www.nat.fau.de/2025/06/17/z...

Mehr Infos: www.nat.fau.de/2025/06/17/z...

