
The Groth lab
@grothlab.bsky.social
The Anja Groth group in Copenhagen studies chromatin replication, epigenetic memory and (epi)genome stability in the context of mitotic cell division
It was wonderful to hear talks from EpiC members @grothlab.bsky.social @nilskrietenstein.bsky.social @nilssonlab.bsky.social & Niels Mailand, now we are settled in our new home @cancer.dk. We are grateful for @dg.dk for this exciting opportunity. Onwards and upwards with EpiC science! 🧬 #CPRLegacy
August 28, 2025 at 5:35 PM
It was wonderful to hear talks from EpiC members @grothlab.bsky.social @nilskrietenstein.bsky.social @nilssonlab.bsky.social & Niels Mailand, now we are settled in our new home @cancer.dk. We are grateful for @dg.dk for this exciting opportunity. Onwards and upwards with EpiC science! 🧬 #CPRLegacy
Thank you, Tae-Kyeong!
March 10, 2025 at 7:22 AM
Thank you, Tae-Kyeong!
Together our work gives a molecular basis for histone supply regulation by CODANIN-1, read the full paper here: rdcu.be/ecbHq . Thanks to the Song lab at KAIST jijoonsong.bsky.social for taking us onboard as collaborators for this exciting new structure-function adventure!
CODANIN-1 sequesters ASF1 by using a histone H3 mimic helix to regulate the histone supply
Nature Communications - CODANIN-1 negatively regulates the function of ASF1, the key chaperone for histone H3-H4 supply. Here the authors present the cryo-EM structure of the CODANIN-1_ASF1A...
rdcu.be
March 6, 2025 at 12:45 PM
Together our work gives a molecular basis for histone supply regulation by CODANIN-1, read the full paper here: rdcu.be/ecbHq . Thanks to the Song lab at KAIST jijoonsong.bsky.social for taking us onboard as collaborators for this exciting new structure-function adventure!
How important is CODANIN/ASF1A complex formation? Inhibiting dimerization in cells or introducing a pathogenic CDA-I mutation in the dimerization interface reduced ASF1A cytoplasmic sequestration. This shows the significance of CODANIN/ASF1A complex formation and possible disease relevance
March 6, 2025 at 12:45 PM
How important is CODANIN/ASF1A complex formation? Inhibiting dimerization in cells or introducing a pathogenic CDA-I mutation in the dimerization interface reduced ASF1A cytoplasmic sequestration. This shows the significance of CODANIN/ASF1A complex formation and possible disease relevance
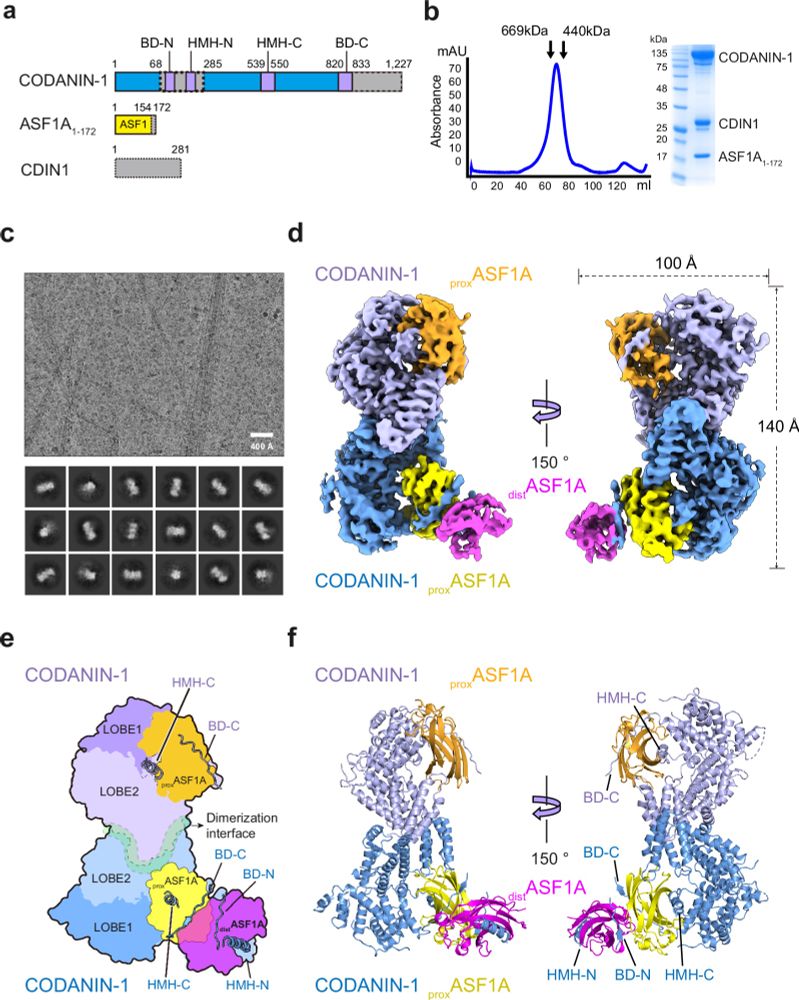
Which CODANIN binding sites are required for ASF1 regulation? Using inducible complementation of CODANIN-1 in cells, we found that mutation of both BD-N and HMH-C is needed to prevent cytoplasmic sequestration of ASF1A. Thus, CODANIN-1 regulates ASF1A nuclear import by this dual-binding mechanism.
March 6, 2025 at 12:45 PM
Which CODANIN binding sites are required for ASF1 regulation? Using inducible complementation of CODANIN-1 in cells, we found that mutation of both BD-N and HMH-C is needed to prevent cytoplasmic sequestration of ASF1A. Thus, CODANIN-1 regulates ASF1A nuclear import by this dual-binding mechanism.
By binding in this way, CODANIN-1 occludes the two major histone-binding surfaces of ASF1A, preventing its chaperone activity. Interestingly, CODANIN-1 could also bind preformed ASF1A/H3-H4 using its B domains, suggesting that it may regulate ASF1 at multiple points in the histone supply chain.
March 6, 2025 at 12:45 PM
By binding in this way, CODANIN-1 occludes the two major histone-binding surfaces of ASF1A, preventing its chaperone activity. Interestingly, CODANIN-1 could also bind preformed ASF1A/H3-H4 using its B domains, suggesting that it may regulate ASF1 at multiple points in the histone supply chain.
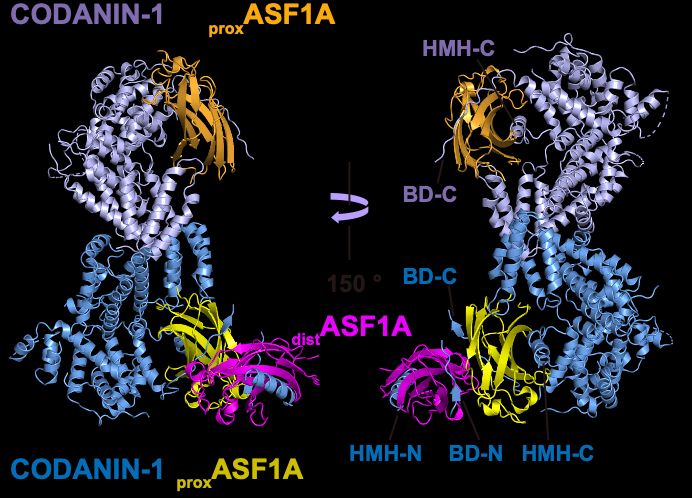
So, how does CODANIN-1 bind ASF1? Our cryo-EM structure reveals that CODANIN-1 forms a dimer, with each monomer binding two ASF1A molecules. These two ASF1As are held in proximal and distal positions by CODANIN’s N-terminal B domain (BD-N) and C-terminal histone H3 mimic helix (HMH-C), respectively.
March 6, 2025 at 12:45 PM
So, how does CODANIN-1 bind ASF1? Our cryo-EM structure reveals that CODANIN-1 forms a dimer, with each monomer binding two ASF1A molecules. These two ASF1As are held in proximal and distal positions by CODANIN’s N-terminal B domain (BD-N) and C-terminal histone H3 mimic helix (HMH-C), respectively.
A bit of background: ASF1 plays a central role in histone nuclear import and supply for replication & transcription. CODANIN-1 is commonly mutated in a rare form of heritable anaemia (CDA-I) and inhibits histone supply by ASF1. But we don’t currently understand these roles on the molecular level.
March 6, 2025 at 12:45 PM
A bit of background: ASF1 plays a central role in histone nuclear import and supply for replication & transcription. CODANIN-1 is commonly mutated in a rare form of heritable anaemia (CDA-I) and inhibits histone supply by ASF1. But we don’t currently understand these roles on the molecular level.
Thank you, Jeroen!
February 20, 2025 at 8:12 AM
Thank you, Jeroen!
If you’re interested in epigenetic inheritance and cell division, check out the vacancies for 6 PhDs and Postdocs positions in our new Center for Epigenetic Cell Memory (EpiC): tinyurl.com/yvd93na2 6/n
6 Postdoctoral and PhD positions in Center for Epigenetic Cell Memory (EpiC)
The Center for Epigenetic Cell Memory (EpiC) is a new Center of Excellence that will be established based on funding by the Danish National Research Foundation.
tinyurl.com
February 19, 2025 at 7:35 PM
If you’re interested in epigenetic inheritance and cell division, check out the vacancies for 6 PhDs and Postdocs positions in our new Center for Epigenetic Cell Memory (EpiC): tinyurl.com/yvd93na2 6/n
Want to dive deeper? 🧐 Read the full paper here: tinyurl.com/233y89d2 🔗📖. Last, a big thank you to all our collaborators and colleagues at #NNF-CPR 🙏. #HistoneRecycling #Epigenetics #Chromatin #ScienceAdvances 5/n
Disabling leading and lagging strand histone transmission results in parental histones loss and reduced cell plasticity and viability
Losing parental histones during DNA replication fork passage challenges differentiation competence and cell viability.
tinyurl.com
February 19, 2025 at 7:35 PM
Want to dive deeper? 🧐 Read the full paper here: tinyurl.com/233y89d2 🔗📖. Last, a big thank you to all our collaborators and colleagues at #NNF-CPR 🙏. #HistoneRecycling #Epigenetics #Chromatin #ScienceAdvances 5/n
Our work highlights 𝐩𝐚𝐫𝐞𝐧𝐭𝐚𝐥 𝐡𝐢𝐬𝐭𝐨𝐧𝐞 𝐫𝐞𝐜𝐲𝐜𝐥𝐢𝐧𝐠 𝐚𝐬 𝐚 𝐤𝐞𝐲 𝐞𝐩𝐢𝐠𝐞𝐧𝐞𝐭𝐢𝐜 𝐬𝐚𝐟𝐞𝐠𝐮𝐚𝐫𝐝 – histone modifications must be 𝐞𝐟𝐟𝐢𝐜𝐢𝐞𝐧𝐭𝐥𝐲 transmitted to 𝐛𝐨𝐭𝐡 daughter strands for this to work! Could failures in this process contribute to developmental disorders, cancer, or aging? More to explore! 4/n
February 19, 2025 at 7:35 PM
Our work highlights 𝐩𝐚𝐫𝐞𝐧𝐭𝐚𝐥 𝐡𝐢𝐬𝐭𝐨𝐧𝐞 𝐫𝐞𝐜𝐲𝐜𝐥𝐢𝐧𝐠 𝐚𝐬 𝐚 𝐤𝐞𝐲 𝐞𝐩𝐢𝐠𝐞𝐧𝐞𝐭𝐢𝐜 𝐬𝐚𝐟𝐞𝐠𝐮𝐚𝐫𝐝 – histone modifications must be 𝐞𝐟𝐟𝐢𝐜𝐢𝐞𝐧𝐭𝐥𝐲 transmitted to 𝐛𝐨𝐭𝐡 daughter strands for this to work! Could failures in this process contribute to developmental disorders, cancer, or aging? More to explore! 4/n
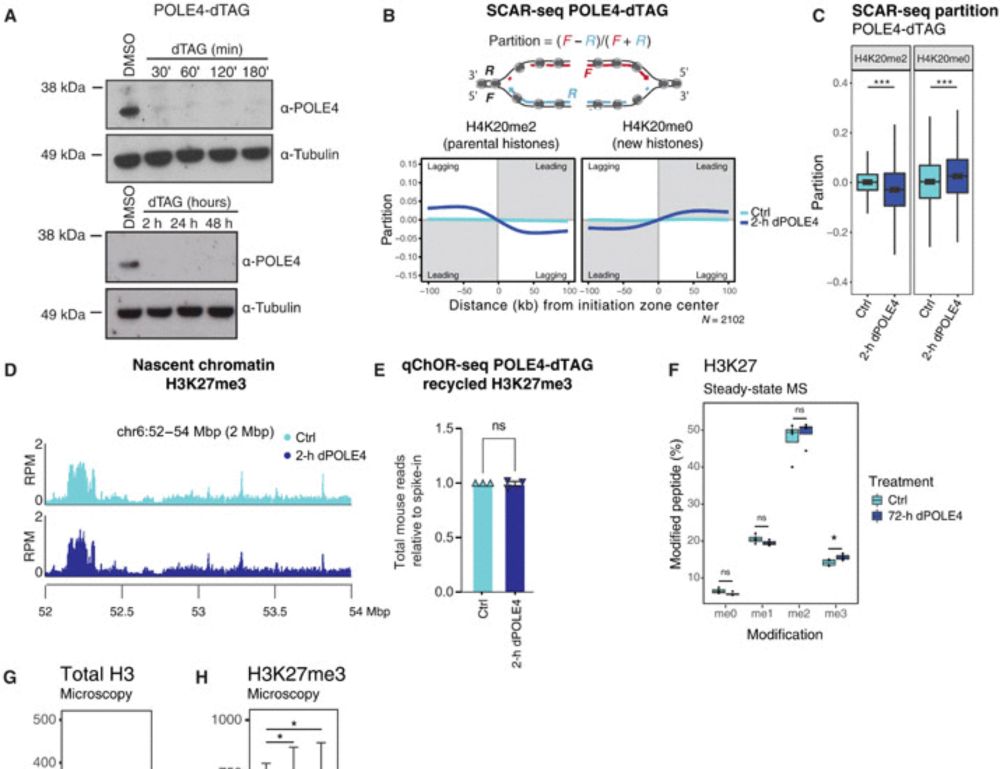
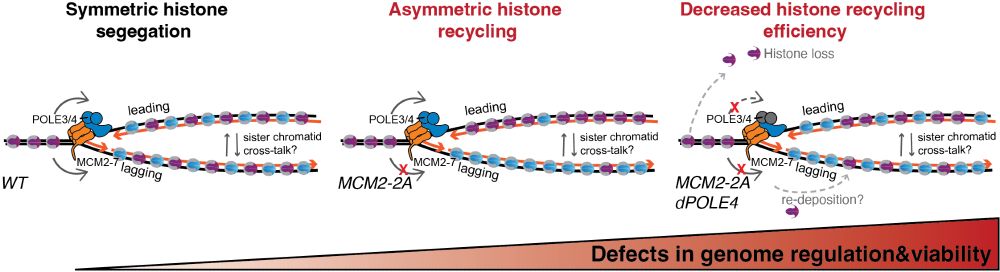
What happens next? Gene misregulation & differentiation defects start to emerge. But when 𝐛𝐨𝐭𝐡 leading & lagging strand recycling are disrupted (POLE4 & MCM2-2A), 𝐬𝐨𝐦𝐞 𝐩𝐚𝐫𝐞𝐧𝐭𝐚𝐥 𝐡𝐢𝐬𝐭𝐨𝐧𝐞𝐬 𝐚𝐫𝐞 𝐥𝐨𝐬𝐭 𝐢𝐧 𝐭𝐫𝐚𝐧𝐬𝐦𝐢𝐬𝐬𝐢𝐨𝐧 —worsening the defects. Unlike single mutants, cells now start to die! 3/n
February 19, 2025 at 7:35 PM
What happens next? Gene misregulation & differentiation defects start to emerge. But when 𝐛𝐨𝐭𝐡 leading & lagging strand recycling are disrupted (POLE4 & MCM2-2A), 𝐬𝐨𝐦𝐞 𝐩𝐚𝐫𝐞𝐧𝐭𝐚𝐥 𝐡𝐢𝐬𝐭𝐨𝐧𝐞𝐬 𝐚𝐫𝐞 𝐥𝐨𝐬𝐭 𝐢𝐧 𝐭𝐫𝐚𝐧𝐬𝐦𝐢𝐬𝐬𝐢𝐨𝐧 —worsening the defects. Unlike single mutants, cells now start to die! 3/n
We use a degron to deplete POLE4, a leading strand recycler, in WT or a background defective in lagging strand recycling (MCM2-2A). The first epigenome alteration upon acute loss of leading strand recycling is 𝐚𝐜𝐜𝐮𝐦𝐮𝐥𝐚𝐭𝐢𝐨𝐧 𝐨𝐟 𝐇𝟑𝐊𝟐𝟕𝐦𝐞𝟑 - 𝐰𝐢𝐭𝐡𝐢𝐧 𝟏-𝟐 𝐜𝐞𝐥𝐥 𝐜𝐲𝐜𝐥𝐞𝐬 - 𝐛𝐞𝐟𝐨𝐫𝐞 𝐭𝐫𝐚𝐧𝐬𝐜𝐫𝐢𝐩𝐭𝐢𝐨𝐧𝐚𝐥 𝐜𝐡𝐚𝐧𝐠𝐞𝐬! 2/n
February 19, 2025 at 7:35 PM
We use a degron to deplete POLE4, a leading strand recycler, in WT or a background defective in lagging strand recycling (MCM2-2A). The first epigenome alteration upon acute loss of leading strand recycling is 𝐚𝐜𝐜𝐮𝐦𝐮𝐥𝐚𝐭𝐢𝐨𝐧 𝐨𝐟 𝐇𝟑𝐊𝟐𝟕𝐦𝐞𝟑 - 𝐰𝐢𝐭𝐡𝐢𝐧 𝟏-𝟐 𝐜𝐞𝐥𝐥 𝐜𝐲𝐜𝐥𝐞𝐬 - 𝐛𝐞𝐟𝐨𝐫𝐞 𝐭𝐫𝐚𝐧𝐬𝐜𝐫𝐢𝐩𝐭𝐢𝐨𝐧𝐚𝐥 𝐜𝐡𝐚𝐧𝐠𝐞𝐬! 2/n

