2022 #CASFutureLeader, 2021 #RealTimeChem Ambassador.
ORCiD: https://orcid.org/0000-0002-2749-8393


(12/12)
(12/12)
(11/n)
(11/n)

But if the potential required is so low, how can this work?? (9/n)

But if the potential required is so low, how can this work?? (9/n)




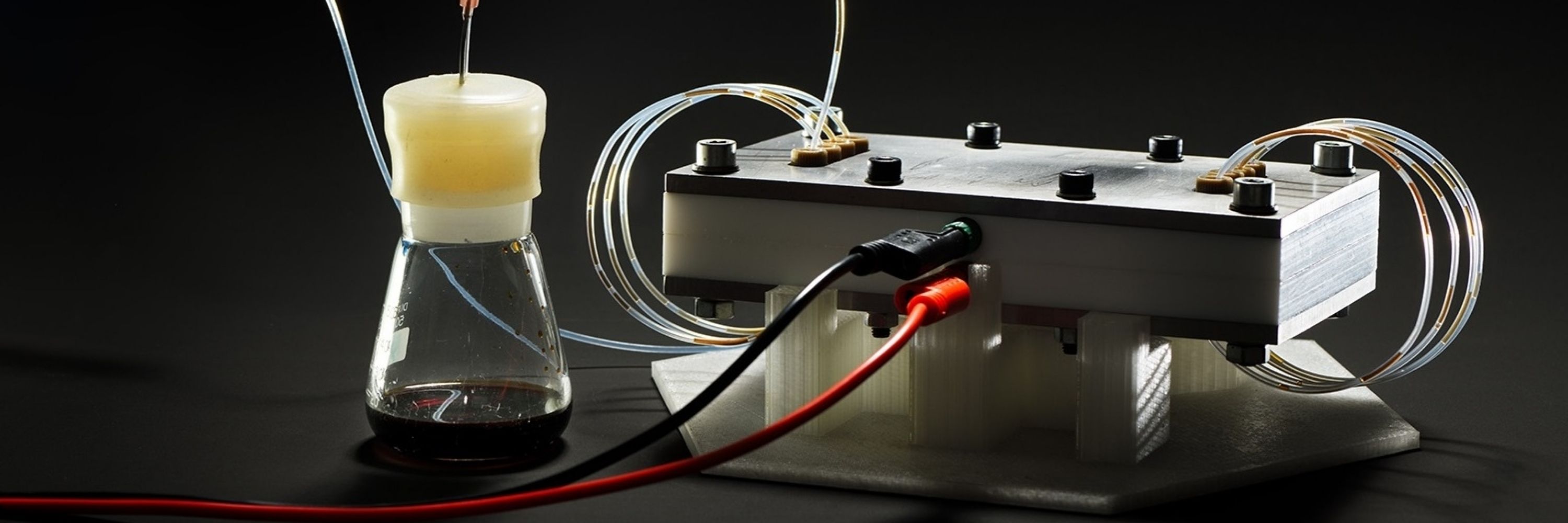
He observed something weird. His Mg sacrificial electrodes were consumed much more than they should have been... (4/n)

He observed something weird. His Mg sacrificial electrodes were consumed much more than they should have been... (4/n)


(12/12)
(12/12)
(11/n)
(11/n)

But if the potential required is so low, how can this work?? (9/n)

But if the potential required is so low, how can this work?? (9/n)

