
https://www.gati-lab.com
https://scholar.google.com/citations?user=5t80YUAAAAAJ&hl=en









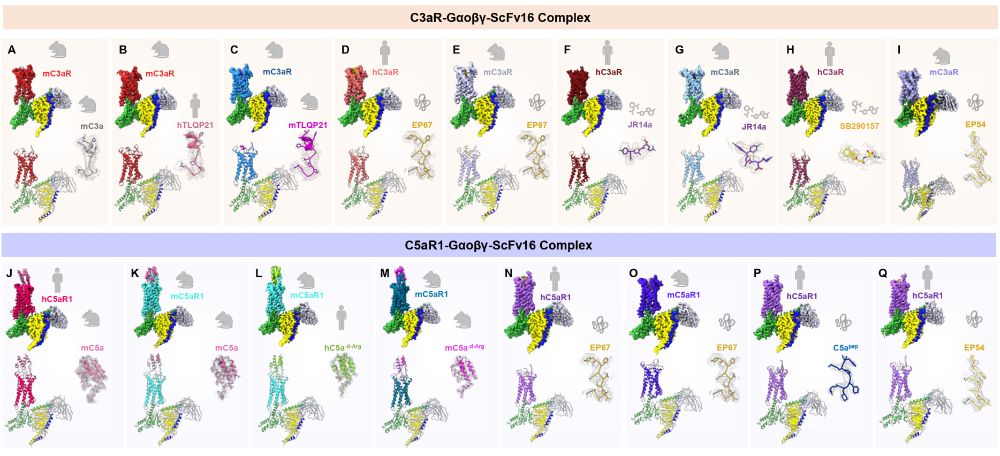




www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

My name is Cornelius Gati and I'm an Assistant Professor at the University of Southern California.
Our lab studies GPCR signaling and pharmacology, with a focus on structural biology (#CryoEM) and biophysics techniques.
I’m excited to get connected with old and new friends!

My name is Cornelius Gati and I'm an Assistant Professor at the University of Southern California.
Our lab studies GPCR signaling and pharmacology, with a focus on structural biology (#CryoEM) and biophysics techniques.
I’m excited to get connected with old and new friends!

