
Chris Peritore-Galve
@fcperitore.bsky.social
Postdoc in the Borden Lacy Lab at Vanderbilt University Medical Center | Incoming Assistant Professor at The Ohio State University | Professional 🦠and 💩 aficionado | Non-professional 🪴🐶🐦 enthusiast| 🇲🇽 🇺🇸 |
20/20 This was a fun and important study, and it was a pleasure to collaborate between the @bordenlacy.bsky.social Lab and our brilliant colleagues at @astra-zeneca.bsky.social!
November 7, 2025 at 7:11 PM
20/20 This was a fun and important study, and it was a pleasure to collaborate between the @bordenlacy.bsky.social Lab and our brilliant colleagues at @astra-zeneca.bsky.social!
19/20 AZD5148 is currently in Phase I clinical trials (NCT06469151), and we are hopeful that this monoclonal antibody will help C. difficile patients in the future.
November 7, 2025 at 7:11 PM
19/20 AZD5148 is currently in Phase I clinical trials (NCT06469151), and we are hopeful that this monoclonal antibody will help C. difficile patients in the future.
18/20 However, the main conclusion is that AZD5148 is a VERY promising, broadly neutralizing mAb that works by itself, in relatively low doses, to prevent severe outcomes of C. diff infection. There is a paucity of therapeutics to combat C. diff infection. And this is likely to be a valuable one.
November 7, 2025 at 7:11 PM
18/20 However, the main conclusion is that AZD5148 is a VERY promising, broadly neutralizing mAb that works by itself, in relatively low doses, to prevent severe outcomes of C. diff infection. There is a paucity of therapeutics to combat C. diff infection. And this is likely to be a valuable one.
17/20 But, from a broader view, we have three different toxin subtypes that cause, in my opinion, vastly different pathologies during infection. Is this due to differences in receptor binding? The substrate targeted? Cell types intoxicated? Or simply toxin titers? Lots of questions remain.
November 7, 2025 at 7:11 PM
17/20 But, from a broader view, we have three different toxin subtypes that cause, in my opinion, vastly different pathologies during infection. Is this due to differences in receptor binding? The substrate targeted? Cell types intoxicated? Or simply toxin titers? Lots of questions remain.
16/20 So interestingly, AZD5148 does not neutralize TcdB3 in vitro, but it protects in vivo. We believe that this is due to the effects TcdB3 has on adherent cells, where, instead of cytopathic rounding, the cells detach from the plate. So the in vitro finding is likely a byproduct of the assay.
November 7, 2025 at 7:11 PM
16/20 So interestingly, AZD5148 does not neutralize TcdB3 in vitro, but it protects in vivo. We believe that this is due to the effects TcdB3 has on adherent cells, where, instead of cytopathic rounding, the cells detach from the plate. So the in vitro finding is likely a byproduct of the assay.
15/20 The lack of outward signs of disease and limited histopathology was curious, so we tested this with additional TcdB3+ strains and found that the mild disease with edema + inflammation being the most informative markers was consistent across ribotype 017 strains.

November 7, 2025 at 7:11 PM
15/20 The lack of outward signs of disease and limited histopathology was curious, so we tested this with additional TcdB3+ strains and found that the mild disease with edema + inflammation being the most informative markers was consistent across ribotype 017 strains.
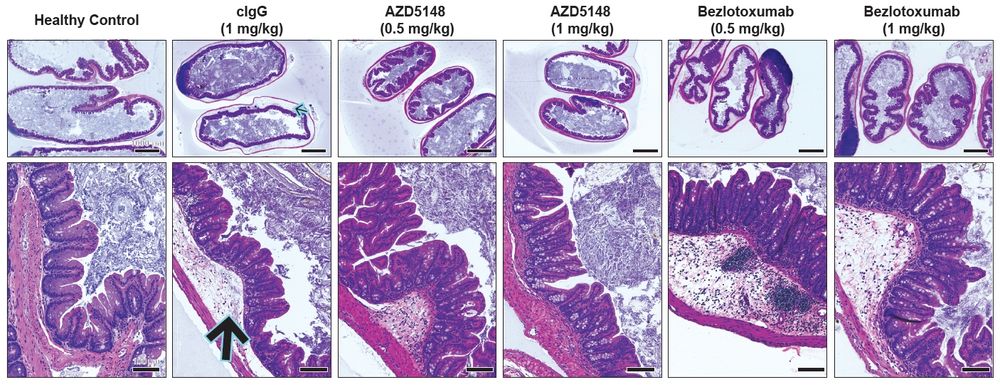
14/20 Mice infected with the TcdB3+ strain develop no appreciable epithelial damage, but they do exhibit high amounts of inflammation and edema. Surprisingly, even though AZD5148 did not neutralize TcdB3 in vitro, it significantly protected against the inflammation and edema phenotypes in vivo!
November 7, 2025 at 7:11 PM
14/20 Mice infected with the TcdB3+ strain develop no appreciable epithelial damage, but they do exhibit high amounts of inflammation and edema. Surprisingly, even though AZD5148 did not neutralize TcdB3 in vitro, it significantly protected against the inflammation and edema phenotypes in vivo!
13/20 Upon initial assessment, it seemed like this strain, despite being isolated from the clinic, did not cause C. difficile infection. There was no diarrhea and minimal weight loss around days 3-6 post-infection. But the tissue tells a different story.
November 7, 2025 at 7:11 PM
13/20 Upon initial assessment, it seemed like this strain, despite being isolated from the clinic, did not cause C. difficile infection. There was no diarrhea and minimal weight loss around days 3-6 post-infection. But the tissue tells a different story.
12/20 What I was really excited about was building up the model of infection with a TcdB3+ strain. This TcdB subtype is typically found in ribotype 017 strains that lack TcdA and are endemic to East Asia. This subtype is also unique in that it preferentially targets Ras-like GTPases.
November 7, 2025 at 7:11 PM
12/20 What I was really excited about was building up the model of infection with a TcdB3+ strain. This TcdB subtype is typically found in ribotype 017 strains that lack TcdA and are endemic to East Asia. This subtype is also unique in that it preferentially targets Ras-like GTPases.
11/20 What is baffling is that despite protecting against lethality and weight loss, mAb therapy did not have an impact on pathology. This could be due to systemic effects of neutralizing TcdB, or the prevention of further damage by directing more AZD5148 from the bloodstream to the tissue, or more.

November 7, 2025 at 7:11 PM
11/20 What is baffling is that despite protecting against lethality and weight loss, mAb therapy did not have an impact on pathology. This could be due to systemic effects of neutralizing TcdB, or the prevention of further damage by directing more AZD5148 from the bloodstream to the tissue, or more.
10/20 AZD5148 was protective down to the low dose of 0.5 mg/kg, whereas bezlotoxumab was most protective at 2.5 mg/kg in our model.
We next tested prophylactic protection during infection with the highly pathogenic VPI 10463 (TcdB1+) strain, and again, AZD5148 outperformed bezlotoxumab.
We next tested prophylactic protection during infection with the highly pathogenic VPI 10463 (TcdB1+) strain, and again, AZD5148 outperformed bezlotoxumab.
November 7, 2025 at 7:11 PM
10/20 AZD5148 was protective down to the low dose of 0.5 mg/kg, whereas bezlotoxumab was most protective at 2.5 mg/kg in our model.
We next tested prophylactic protection during infection with the highly pathogenic VPI 10463 (TcdB1+) strain, and again, AZD5148 outperformed bezlotoxumab.
We next tested prophylactic protection during infection with the highly pathogenic VPI 10463 (TcdB1+) strain, and again, AZD5148 outperformed bezlotoxumab.
9/20 Using AZD5148 in a prophylactic model, we found that AZD5148 alone was sufficient to protect against severe C. difficile infection with a TcdB2+ strain. The addition of a TcdA-neutralizing mAb, PA50, added no benefit over AZD5148 alone, which tracks with data from bezlotoxumab clinical trials.
www.nejm.org
November 7, 2025 at 7:11 PM
9/20 Using AZD5148 in a prophylactic model, we found that AZD5148 alone was sufficient to protect against severe C. difficile infection with a TcdB2+ strain. The addition of a TcdA-neutralizing mAb, PA50, added no benefit over AZD5148 alone, which tracks with data from bezlotoxumab clinical trials.
8/20 In vitro, AZD5148 potently neutralized TcdB1 and TcdB2 with EC50 values 1,000- or 14,000-fold lower than those of bezlotoxumab, respectively. However, AZD5148 was unable to neutralize TcdB3 in vitro, which contains the Y323H mutation in AZD5148's epitope...
November 7, 2025 at 7:11 PM
8/20 In vitro, AZD5148 potently neutralized TcdB1 and TcdB2 with EC50 values 1,000- or 14,000-fold lower than those of bezlotoxumab, respectively. However, AZD5148 was unable to neutralize TcdB3 in vitro, which contains the Y323H mutation in AZD5148's epitope...
7/20 So we set off to test whether AZD5148 would protect against diverse C. difficile strains that encode TcdB1, TcdB2, and TcdB3 variants. We also compared its efficacy to bezlotoxumab (Zinplava), the existing mAb that was discontinued in January.
November 7, 2025 at 7:11 PM
7/20 So we set off to test whether AZD5148 would protect against diverse C. difficile strains that encode TcdB1, TcdB2, and TcdB3 variants. We also compared its efficacy to bezlotoxumab (Zinplava), the existing mAb that was discontinued in January.
6/20 Around the same time, the field gained a deeper appreciation of divergence in TcdB sequences, with the TcdB1, TcdB2, and TcdB3 subtypes being the most clinically-relevant subtypes.
www.nature.com/articles/s42...
journals.plos.org/plospathogen...
www.nature.com/articles/s42...
journals.plos.org/plospathogen...
November 7, 2025 at 7:11 PM
6/20 Around the same time, the field gained a deeper appreciation of divergence in TcdB sequences, with the TcdB1, TcdB2, and TcdB3 subtypes being the most clinically-relevant subtypes.
www.nature.com/articles/s42...
journals.plos.org/plospathogen...
www.nature.com/articles/s42...
journals.plos.org/plospathogen...
5/20 But one of the key findings was that some strains encode a TcdB sequence variant that contains a mutation in AZD5148's epitope, which significantly decreased binding affinity...
November 7, 2025 at 7:11 PM
5/20 But one of the key findings was that some strains encode a TcdB sequence variant that contains a mutation in AZD5148's epitope, which significantly decreased binding affinity...
4/20 Members of the @bordenlacy.bsky.social Lab then did some fantastic structural and mechanistic work to show that PA41/AZD5148 binds the TcdB enzymatic domain and blocks the translocation of this toxic cargo from the endosomal pore into the host cytosol. www.jbc.org/article/S002...
November 7, 2025 at 7:11 PM
4/20 Members of the @bordenlacy.bsky.social Lab then did some fantastic structural and mechanistic work to show that PA41/AZD5148 binds the TcdB enzymatic domain and blocks the translocation of this toxic cargo from the endosomal pore into the host cytosol. www.jbc.org/article/S002...
3/20 Past work generated the novel anti-TcdB antibody PA41 (now called AZD5148), which neutralized TcdB with picomolar affinity and protected hamsters against lethal infection when combined with an anti-TcdA neutralizing antibody, PA50. academic.oup.com/jid/article/...
November 7, 2025 at 7:11 PM
3/20 Past work generated the novel anti-TcdB antibody PA41 (now called AZD5148), which neutralized TcdB with picomolar affinity and protected hamsters against lethal infection when combined with an anti-TcdA neutralizing antibody, PA50. academic.oup.com/jid/article/...
2/20 C. difficile infection is driven by two protein toxins, TcdA and TcdB. However, years of work suggest that TcdB is the main virulence factor.
The only FDA-approved anti-TcdB mAb, bezlotoxumab (Zinplava) for the prevention of recurrent C. difficile infection, was discontinued earlier this year.
The only FDA-approved anti-TcdB mAb, bezlotoxumab (Zinplava) for the prevention of recurrent C. difficile infection, was discontinued earlier this year.
www.reuters.com
November 7, 2025 at 7:11 PM
2/20 C. difficile infection is driven by two protein toxins, TcdA and TcdB. However, years of work suggest that TcdB is the main virulence factor.
The only FDA-approved anti-TcdB mAb, bezlotoxumab (Zinplava) for the prevention of recurrent C. difficile infection, was discontinued earlier this year.
The only FDA-approved anti-TcdB mAb, bezlotoxumab (Zinplava) for the prevention of recurrent C. difficile infection, was discontinued earlier this year.

