
Eszter Posfai
@eposfai.bsky.social
Preimplantation development and germline biology. Live imaging and genome engineering. Assistant Prof at Princeton.
Moral of the story: the mouse embryo uses some preplanning as well as some randomness to conduct this important fate decision. Huge thank you to everyone involved + to @currentbiology.bsky.social for the awesome publishing experience! www.cell.com/current-biol...
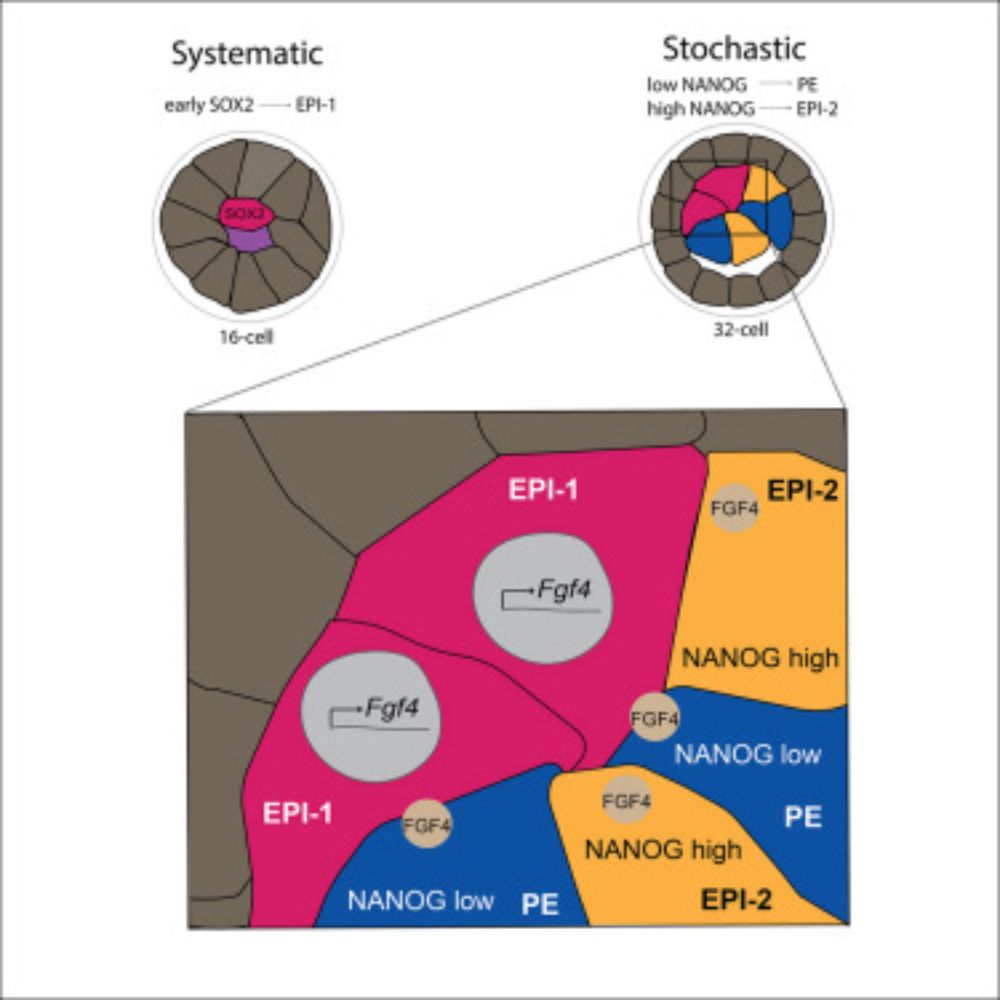
Live imaging endogenous transcription factor dynamics reveals mechanisms of epiblast and primitive endoderm fate segregation
Kim-Yip, Denberg, et al. visualize epiblast and primitive endoderm specification by
live imaging key transcription factors. They reveal systematic and stochastic features
by identifying a primary epib...
www.cell.com
August 2, 2025 at 7:31 PM
Moral of the story: the mouse embryo uses some preplanning as well as some randomness to conduct this important fate decision. Huge thank you to everyone involved + to @currentbiology.bsky.social for the awesome publishing experience! www.cell.com/current-biol...
Whether the trajectory is maintained towards PE or switches to Epi is in part predicted by the level of another TF, NANOG, which in turn seems to behave in a stochastic manner.
August 2, 2025 at 7:31 PM
Whether the trajectory is maintained towards PE or switches to Epi is in part predicted by the level of another TF, NANOG, which in turn seems to behave in a stochastic manner.
We then showed that FGF signaling, with the ligand likely from the primary Epi lineage, sets all other cells on the course towards PE differentiation. This trajectory however switches in some lineages, within a defined developmental window, giving rise to a second wave of Epi cells.
August 2, 2025 at 7:31 PM
We then showed that FGF signaling, with the ligand likely from the primary Epi lineage, sets all other cells on the course towards PE differentiation. This trajectory however switches in some lineages, within a defined developmental window, giving rise to a second wave of Epi cells.
We found the emergence of a founder, termed primary Epi cell lineage as the first symmetry breaking event. Moreover, this primary Epi cell was marked by early expression of SOX2, a TF linked to the segregation of the previous fate decision. Therefore, symmetry breaking is linked to lineage history.
August 2, 2025 at 7:31 PM
We found the emergence of a founder, termed primary Epi cell lineage as the first symmetry breaking event. Moreover, this primary Epi cell was marked by early expression of SOX2, a TF linked to the segregation of the previous fate decision. Therefore, symmetry breaking is linked to lineage history.
We simultaneously live image endogenously tagged transcription factors (up to 3 of them!) key to this fate decision, which allowed us not only to analyze the lineage history of cells, but also to directly visualize how individual cells choose their fates in developing embryos.
August 2, 2025 at 7:31 PM
We simultaneously live image endogenously tagged transcription factors (up to 3 of them!) key to this fate decision, which allowed us not only to analyze the lineage history of cells, but also to directly visualize how individual cells choose their fates in developing embryos.
The second paper, incorporating a probabilistic modeling approach, investigates the relationship between YAP dynamics and two of its downstream transcription factor targets, SOX2 (ICM) and CDX2 (TE).
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

Generative model for the first cell fate bifurcation in mammalian development
The first cell fate bifurcation in mammalian development directs cells toward either the trophectoderm (TE) or inner cell mass (ICM) compartments in preimplantation embryos. This decision is regulated by the subcellular localization of a transcriptional co-activator YAP and takes place over several progressively asyn-chronous cleavage divisions. As a result of this asynchrony and variable arrangement of blastomeres, reconstructing the dynamics of the TE/ICM cell specification from fixed embryos is extremely challenging. To address this, we developed a live imaging approach and applied it to measure pairwise dynamics of nuclear YAP and its direct target genes, CDX2 and SOX2, key transcription factors of TE and ICM, respectively. Using these datasets, we constructed a generative model of the first cell fate bifurcation, which reveals the time-dependent statistics of the TE and ICM cell allocation. In addition to making testable predictions for the joint dynamics of the full YAP/CDX2/SOX2 motif, the model revealed the stochastic nature of the induction timing of the key cell fate determinants and identified the features of YAP dynamics that are necessary or sufficient for this induction. Notably, temporal heterogeneity was particularly prominent for SOX2 expression among ICM cells. As heterogeneities within the ICM have been linked to the initiation of the second cell fate decision in the embryo, understanding the origins of this variability is of key significance. The presented approach reveals the dynamics of the first cell fate choice and lays the groundwork for dissecting the next cell fate bifurcations in mouse development. ### Competing Interest Statement The authors have declared no competing interest.
www.biorxiv.org
March 5, 2025 at 2:54 AM
The second paper, incorporating a probabilistic modeling approach, investigates the relationship between YAP dynamics and two of its downstream transcription factor targets, SOX2 (ICM) and CDX2 (TE).
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...
The first paper looks at how different inputs, such as cell position and shape, cell cycle progression and transitioning from maternal to zygotic resources during early development impact the nuclear levels of YAP, a key regulator this fate decision.
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

Geometric, cell cycle and maternal-to-zygotic transition-associated YAP dynamics during preimplantation embryo development
During the first cell fate decision in mammalian embryos the inner cell mass cells, which will give rise to the embryo proper and other extraembryonic tissues, segregate from the trophectoderm cells, the precursors of the placenta. Cell fate segregation proceeds in a gradual manner encompassing two rounds of cell division, as well as cell positional and morphological changes. While it is known that the activity of the Hippo signaling pathway and the subcellular localization of its downstream effector YAP dictate lineage specific gene expression, the response of YAP to these dynamic cellular changes remains incompletely understood. Here we address these questions by quantitative live imaging of endogenously tagged YAP while simultaneously monitoring geometric cellular features and cell cycle progression throughout cell fate segregation. We apply a probabilistic model to our dynamic data, providing a quantitative characterization of the mutual effects of YAP and cellular relative exposed area, which has previously been shown to correlate with subcellular YAP localization in fixed samples. Additionally, we study how nuclear YAP levels are influenced by other factors, such as the decreasing pool of maternally provided YAP that is partitioned to daughter cells through cleavage divisions, cell cycle-associated nuclear volume changes, and a delay after divisions in adjusting YAP levels to new cell positions. Interestingly, we find that establishing low nuclear YAP levels required for the inner cell mass fate is largely achieved by passive cell cycle-associated mechanisms. Moreover, contrary to expectations, we find that mechanical perturbations that result in cell shape changes do not influence YAP localization in the embryo. Together our work identifies how various inputs are integrated over a dynamic developmental time course to shape the levels of a key molecular determinant of the first cell fate choice. ### Competing Interest Statement The authors have declared no competing interest.
www.biorxiv.org
March 5, 2025 at 2:54 AM
The first paper looks at how different inputs, such as cell position and shape, cell cycle progression and transitioning from maternal to zygotic resources during early development impact the nuclear levels of YAP, a key regulator this fate decision.
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...
Congrats, looking fwd to reading!!
February 12, 2025 at 12:18 AM
Congrats, looking fwd to reading!!

