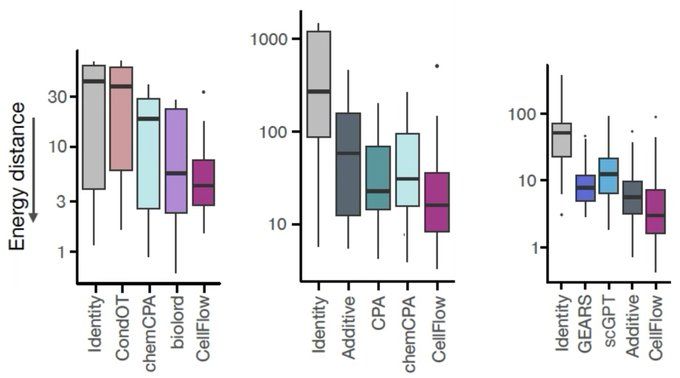
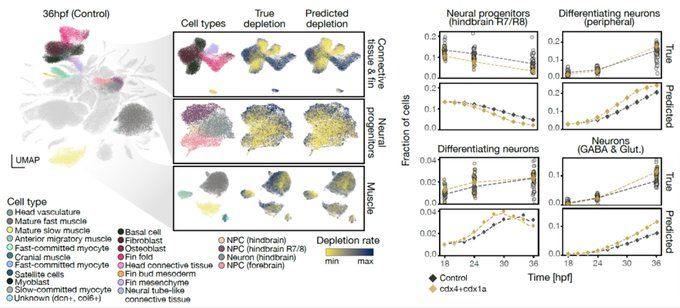
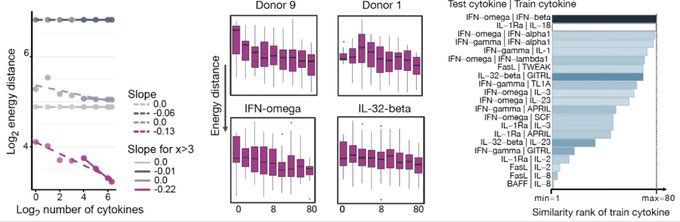
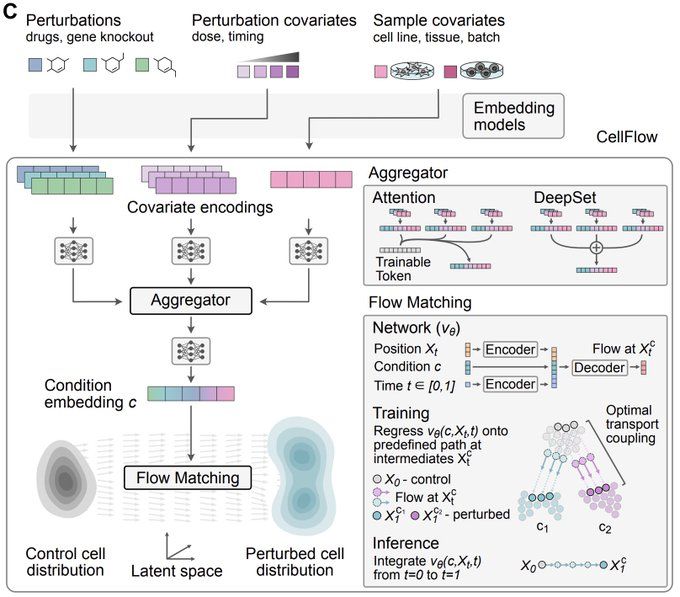
bsky.app/profile/josc...

bsky.app/profile/josc...
Head to cellflow.readthedocs.io for tutorials, and get in touch—Plenty of exciting directions to explore!
Head to cellflow.readthedocs.io for tutorials, and get in touch—Plenty of exciting directions to explore!