
Thanks a lot to @chemieverband.bsky.social for the generous funding. pubs.acs.org/doi/10.1021/...

Thanks a lot to @chemieverband.bsky.social for the generous funding. pubs.acs.org/doi/10.1021/...
pubs.rsc.org/en/content/a...
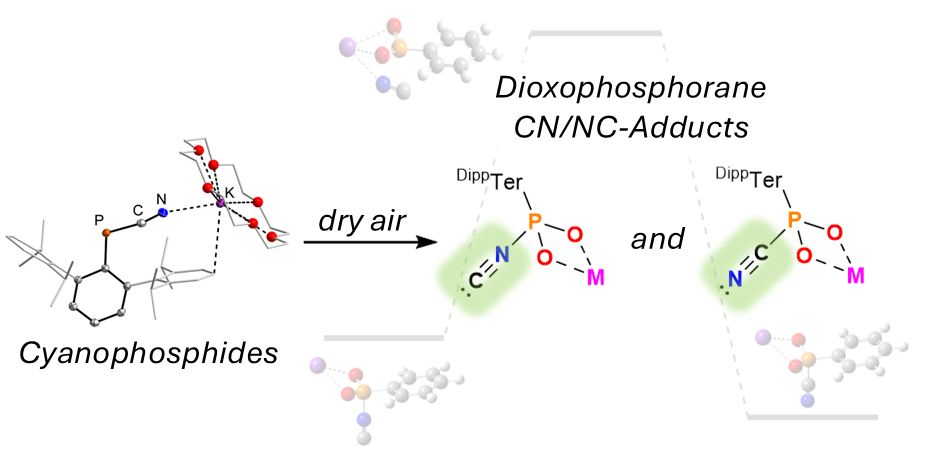
Also thanks @pchem.bsky.social for being on the committee. #phdone #proudpi #31p

Also thanks @pchem.bsky.social for being on the committee. #phdone #proudpi #31p

Also thanks @pchem.bsky.social for being on the committee. #phdone #proudpi #31p
pubs.acs.org/doi/10.1021/...
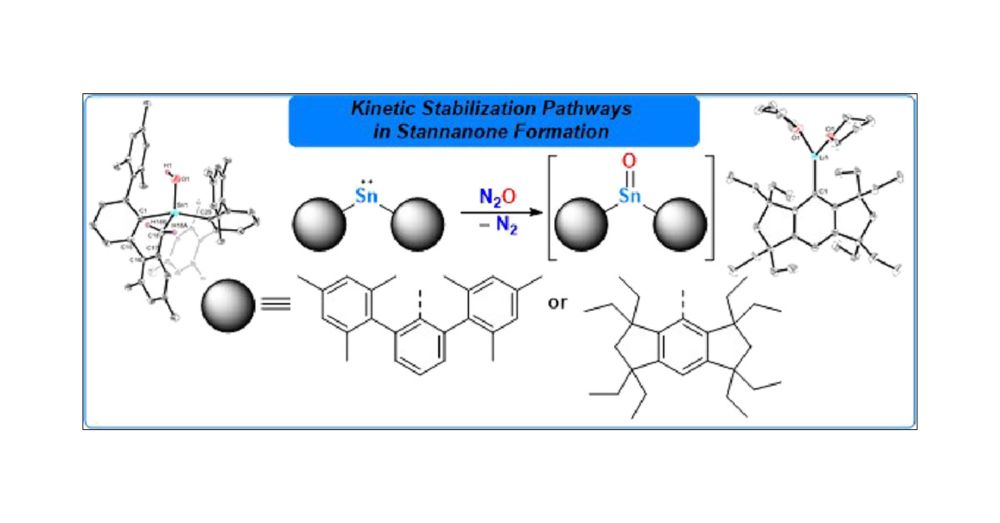
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/full/10....
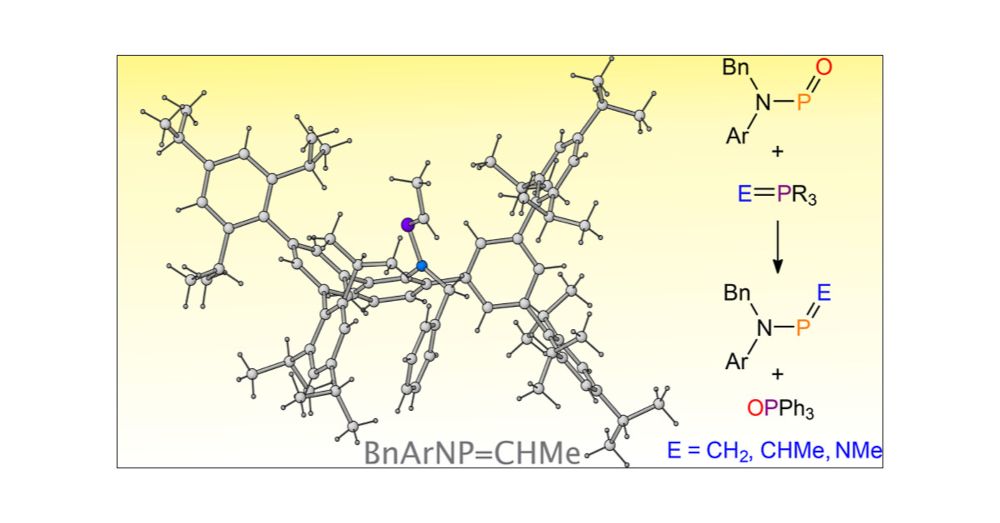
pubs.acs.org/doi/full/10....
Tim’s newest work on the reactivity of phosphaalumenes is out. When P=Al meets it’s smaller sibling a unique B,N,Al,P-heterocycle is formed. Congrats Tim and Leonie!
#chemsky @chemcomm.bsky.social @likat.bsky.social #31p
pubs.rsc.org/en/content/a...
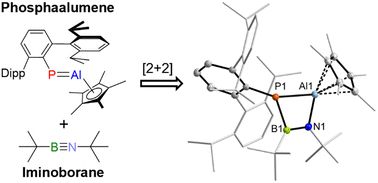
Tim’s newest work on the reactivity of phosphaalumenes is out. When P=Al meets it’s smaller sibling a unique B,N,Al,P-heterocycle is formed. Congrats Tim and Leonie!
#chemsky @chemcomm.bsky.social @likat.bsky.social #31p
pubs.rsc.org/en/content/a...
pubs.acs.org/doi/10.1021/...
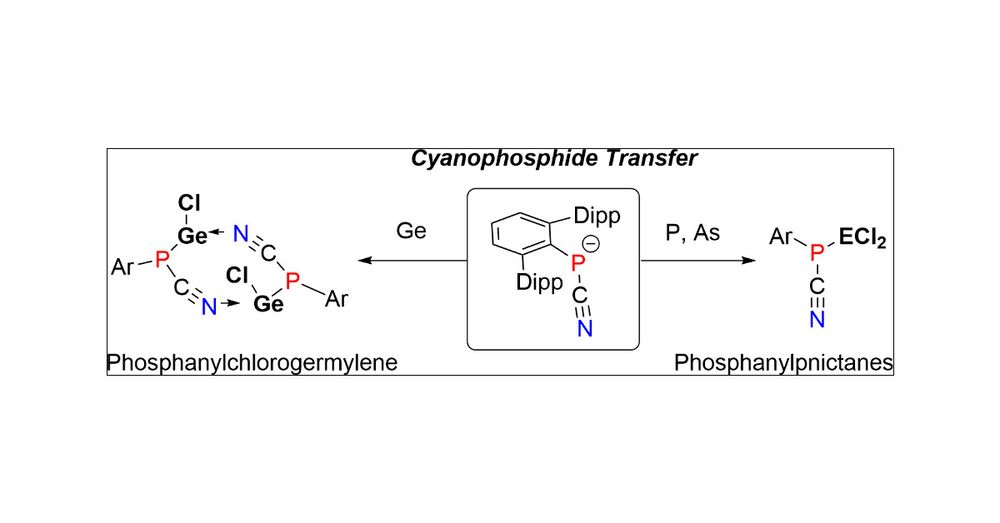
pubs.acs.org/doi/10.1021/...
pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...
Also a big shout out to the organizers Fabian Dielmann, Stephan Hohloch and their staff for organizing a wonderful conference!
#ewpc21 #31p #chemsky @likat.bsky.social

Also a big shout out to the organizers Fabian Dielmann, Stephan Hohloch and their staff for organizing a wonderful conference!
#ewpc21 #31p #chemsky @likat.bsky.social

