
Utilization of an attached organic chromophore while maintaining a luminescent and photoactive LMCT excited state
Joel Wellauer & @bjoernpfund.bsky.social in JACS
pubs.acs.org/doi/10.1021/...
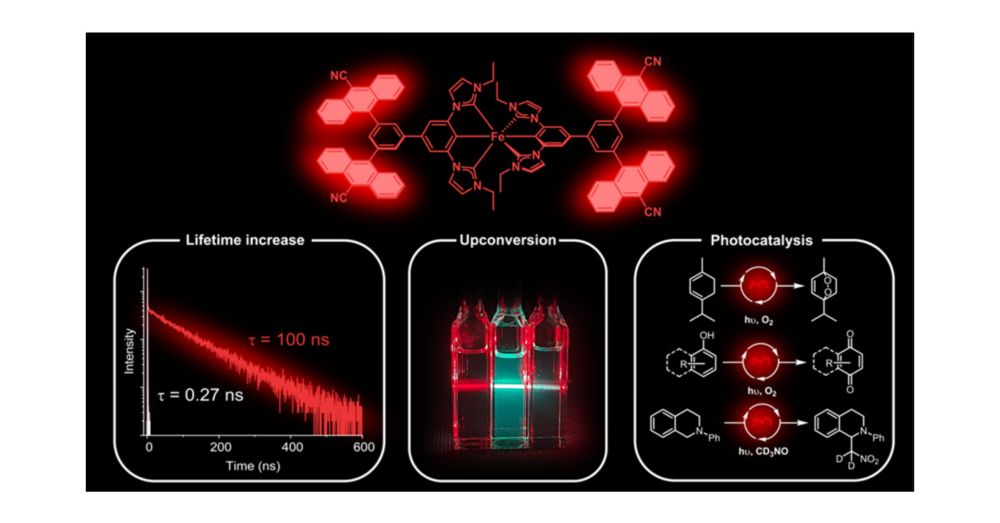
Utilization of an attached organic chromophore while maintaining a luminescent and photoactive LMCT excited state
Joel Wellauer & @bjoernpfund.bsky.social in JACS
pubs.acs.org/doi/10.1021/...
With Giacomo Morselli and Christian Reber in JACS @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
With Giacomo Morselli and Christian Reber in JACS @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...

