
Read more: www.medpagetoday.com/washington-w...
drive.google.com/file/d/1NhrG...
drive.google.com/file/d/1NhrG...




mailchi.mp/eb7d1808132b...

mailchi.mp/eb7d1808132b...

www.healthaffairs.org/do/10.1377/f...
www.healthaffairs.org/do/10.1377/f...
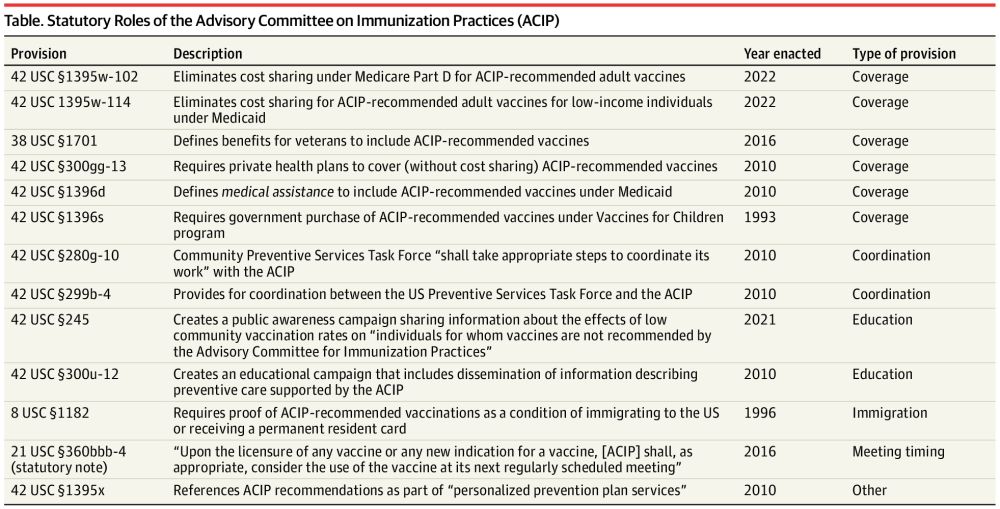






We've sent it out, so law review folks- check your inboxes! #lawsky
papers.ssrn.com/sol3/papers....

We've sent it out, so law review folks- check your inboxes! #lawsky
papers.ssrn.com/sol3/papers....
- the promise and risk of drug repurposing,
- drug importation to address shortages,
- use of subgroup analysis in cost-effectiveness studies
- biosimilar patent litigation,
- and much more!
#medsky #healthpolicy

- the promise and risk of drug repurposing,
- drug importation to address shortages,
- use of subgroup analysis in cost-effectiveness studies
- biosimilar patent litigation,
- and much more!
#medsky #healthpolicy


