
一点浩然气 千里快哉风
airmovingdevice@protonmail.com
这两项规定明显弱于之前征求意见稿中的硬性规定:首个中选周期内不得进行重要生产环节的变更,否则取消中选资格。
大概是多方博弈的结果,baby steps也好吧。


这两项规定明显弱于之前征求意见稿中的硬性规定:首个中选周期内不得进行重要生产环节的变更,否则取消中选资格。
大概是多方博弈的结果,baby steps也好吧。
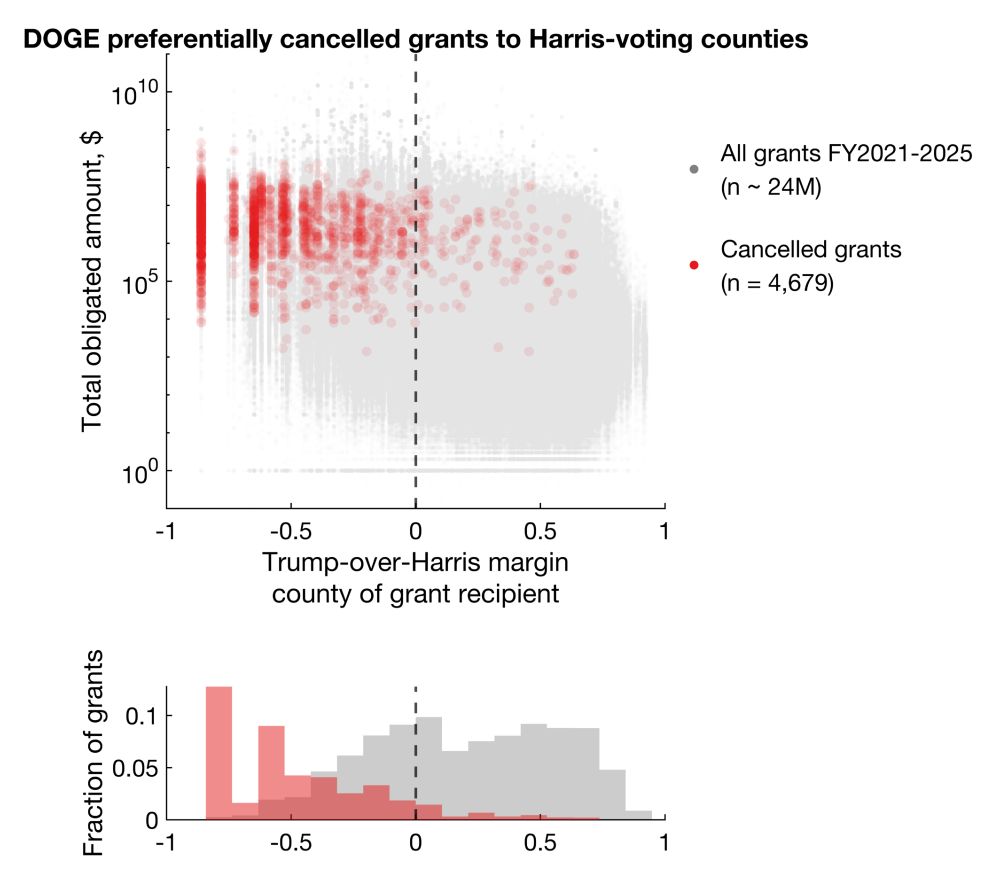
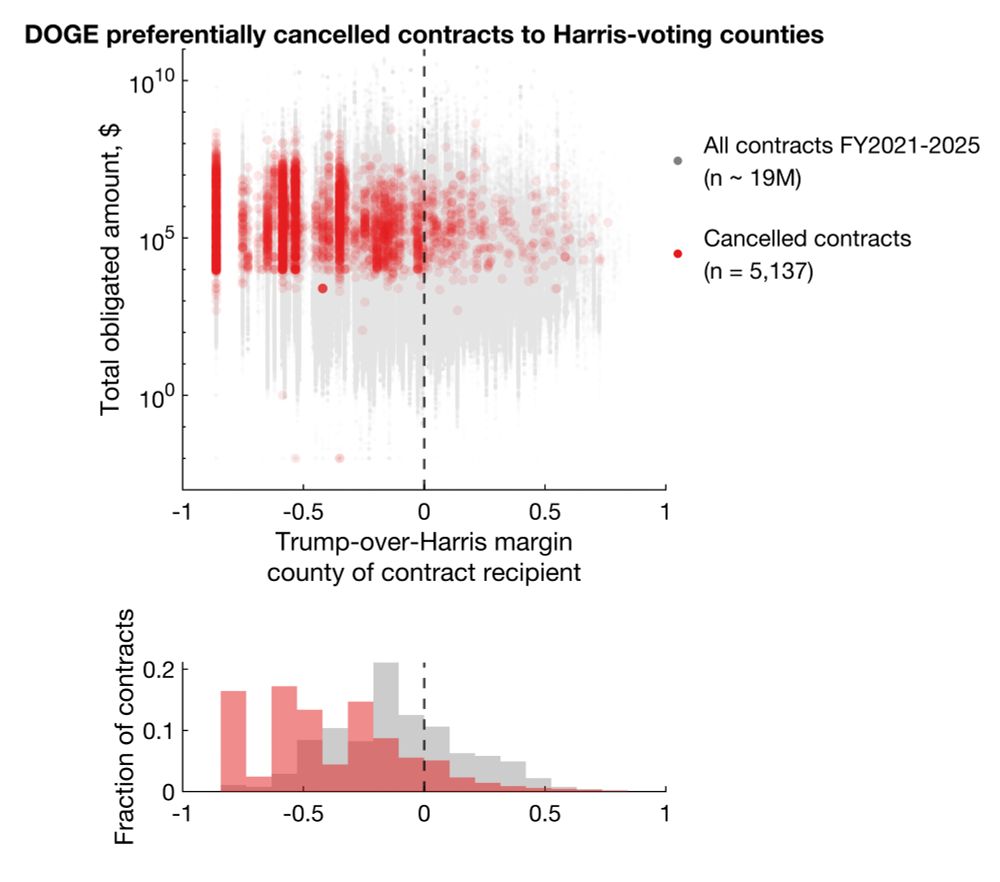
Statistically significant differences shown with Mann-Whitney and Kolmogorov-Smirnov tests (p < 1e-100).
Large cluster on very left is DC.
Statistically significant differences shown with Mann-Whitney and Kolmogorov-Smirnov tests (p < 1e-100).
Large cluster on very left is DC.
Clearly, there's a bias for more cancellations in Harris counties. But does this reflect true bias or simply more contracts/grants awarded to Harris counties?
Clearly, there's a bias for more cancellations in Harris counties. But does this reflect true bias or simply more contracts/grants awarded to Harris counties?
I then cross-referenced them to official spending data on USAspending using links provided by DOGE and ended up with 5,137 and 4,679 contracts and grants with rich metadata.
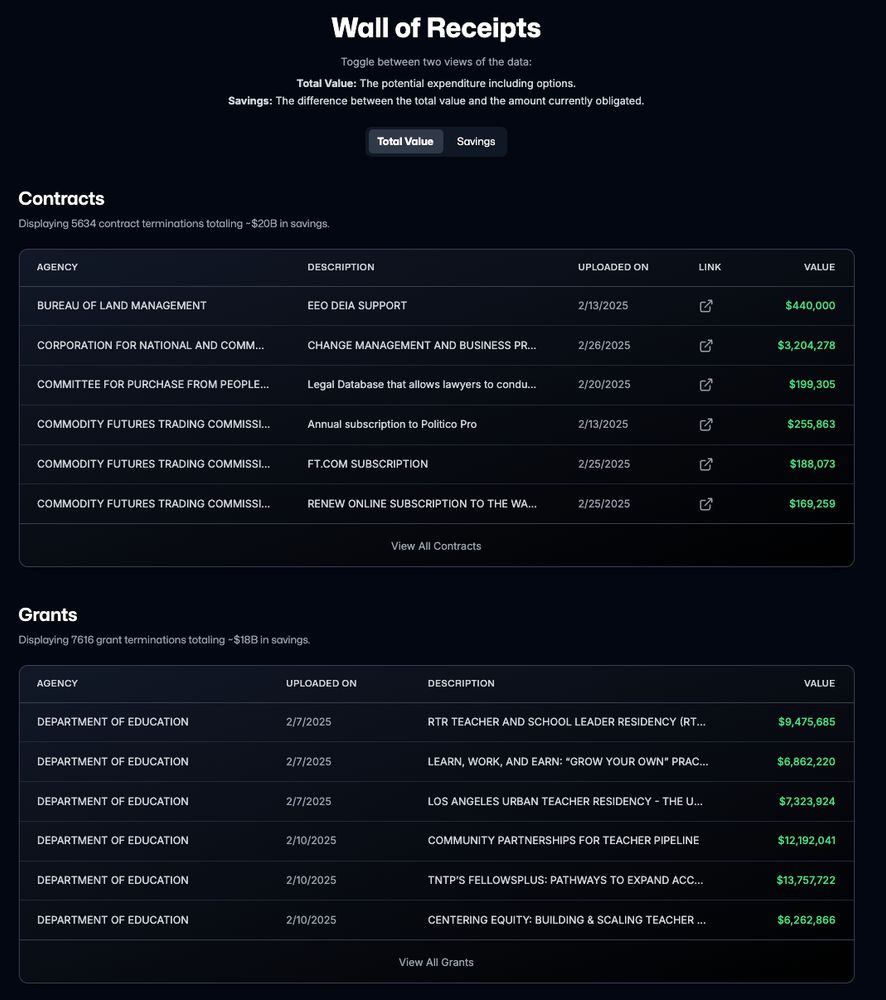
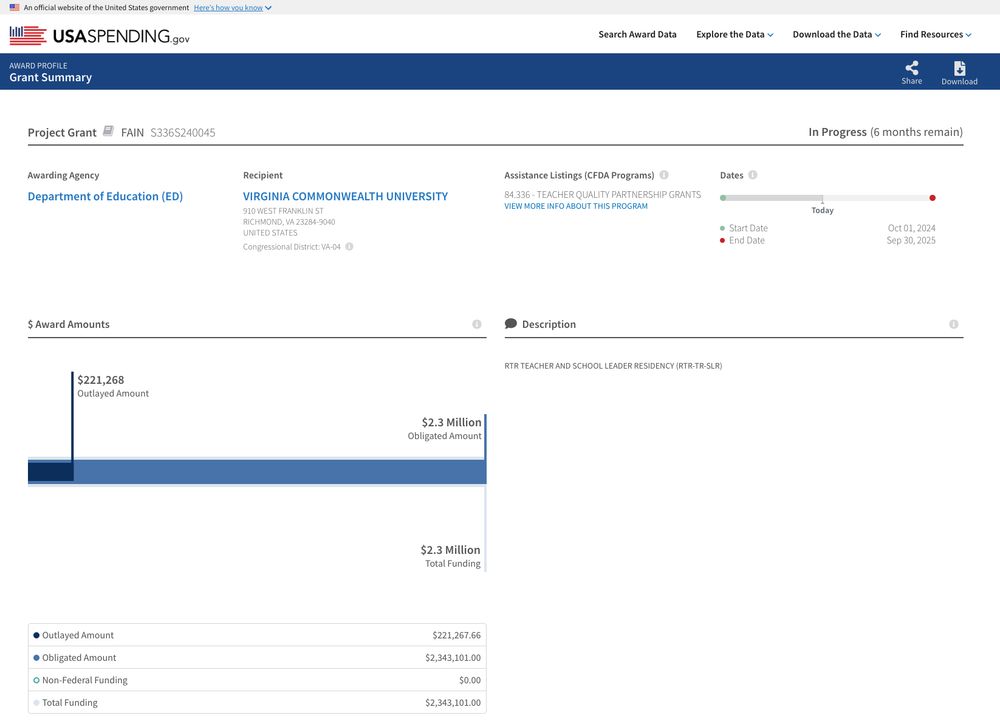
I then cross-referenced them to official spending data on USAspending using links provided by DOGE and ended up with 5,137 and 4,679 contracts and grants with rich metadata.
Among cancellations with election data available, 92.9% and 86.1% cancelled grants and contracts went to Harris counties, representing 96.6% and 92.4% of total dollar amounts.
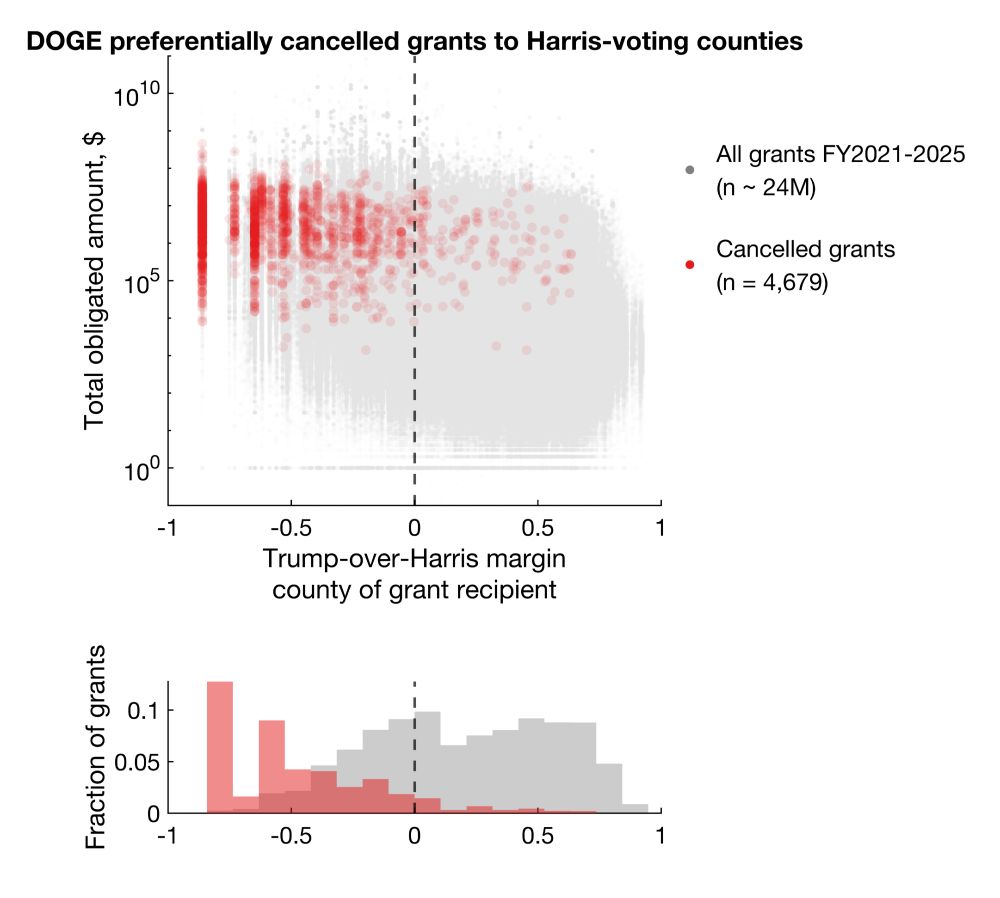
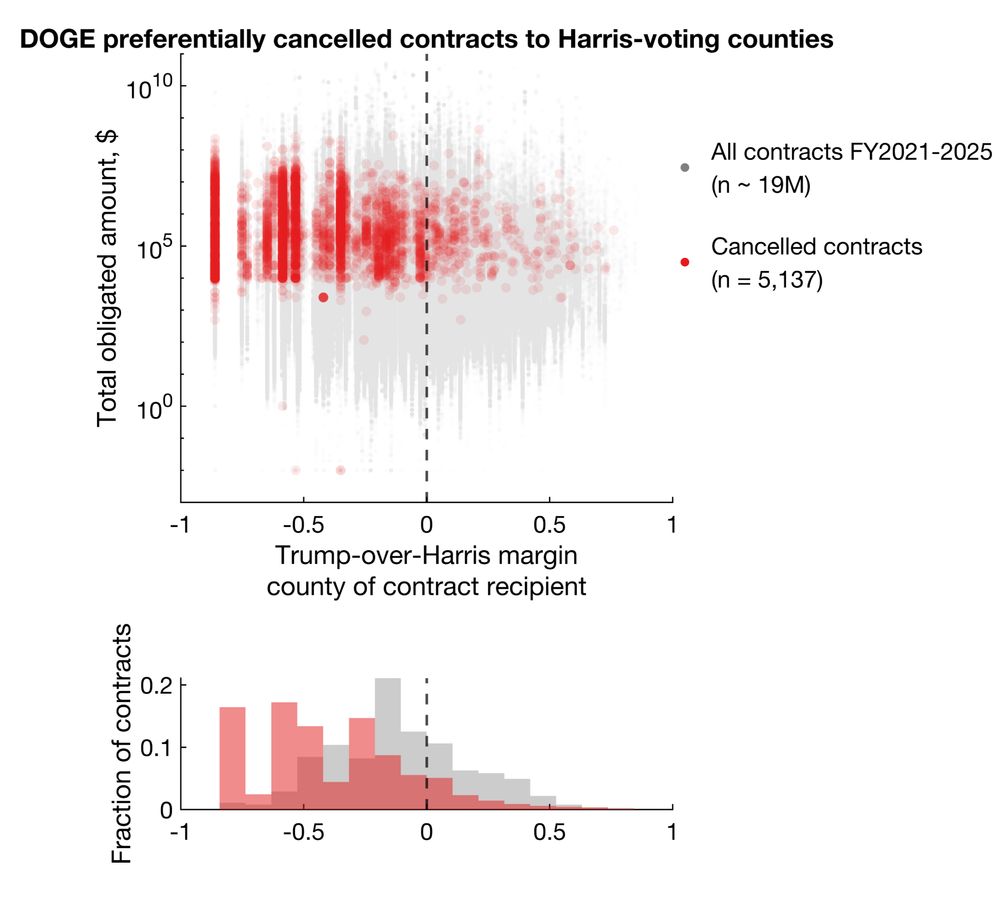
Among cancellations with election data available, 92.9% and 86.1% cancelled grants and contracts went to Harris counties, representing 96.6% and 92.4% of total dollar amounts.
While the number of total filings were similar between the two groups, production-related changes in jicai drugs were ~2-fold that of non-jicai.
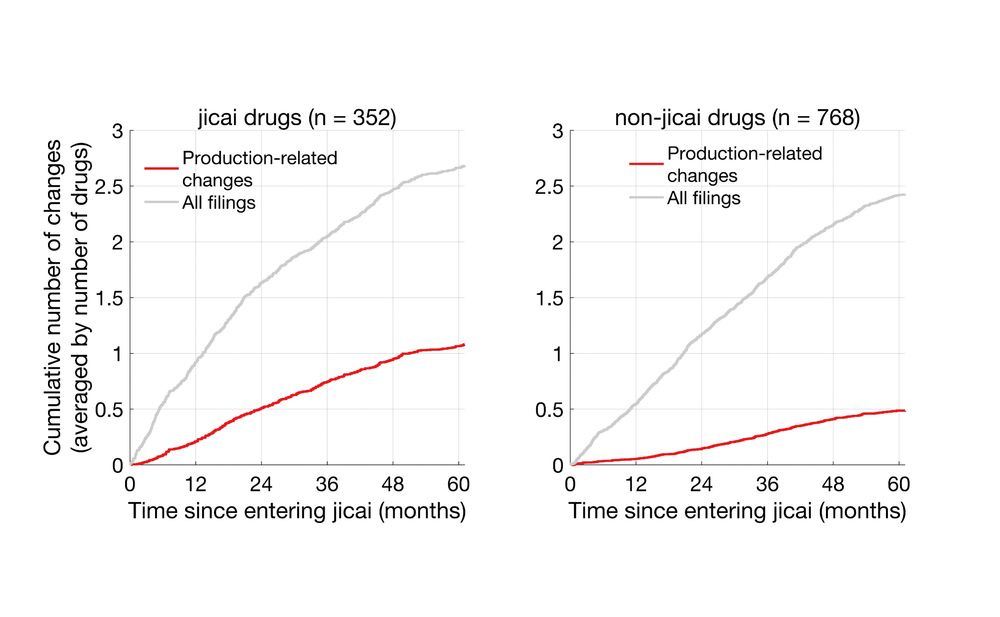
While the number of total filings were similar between the two groups, production-related changes in jicai drugs were ~2-fold that of non-jicai.
Clearly, Telmisartan generics that entered jicai underwent more production-related changes than non-jicai generics.
Same trends were also seen for metformin hydrochloride generics.
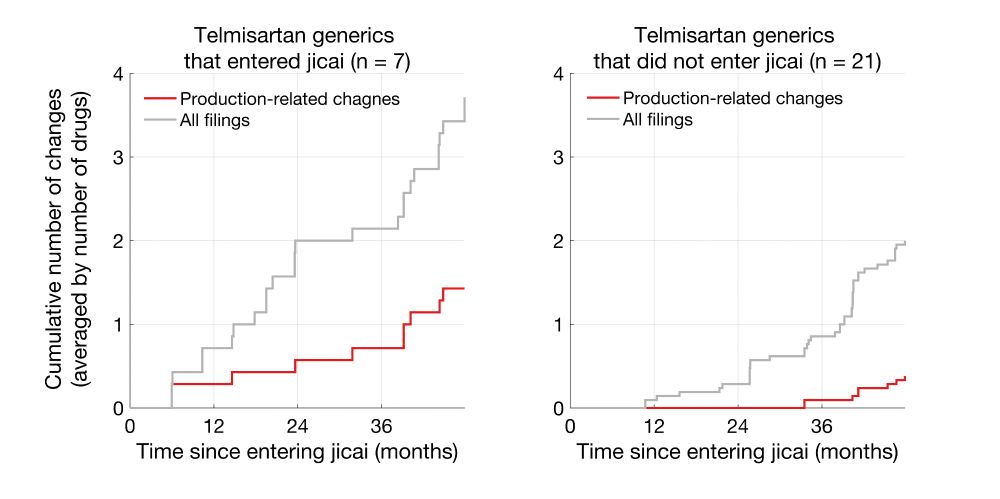
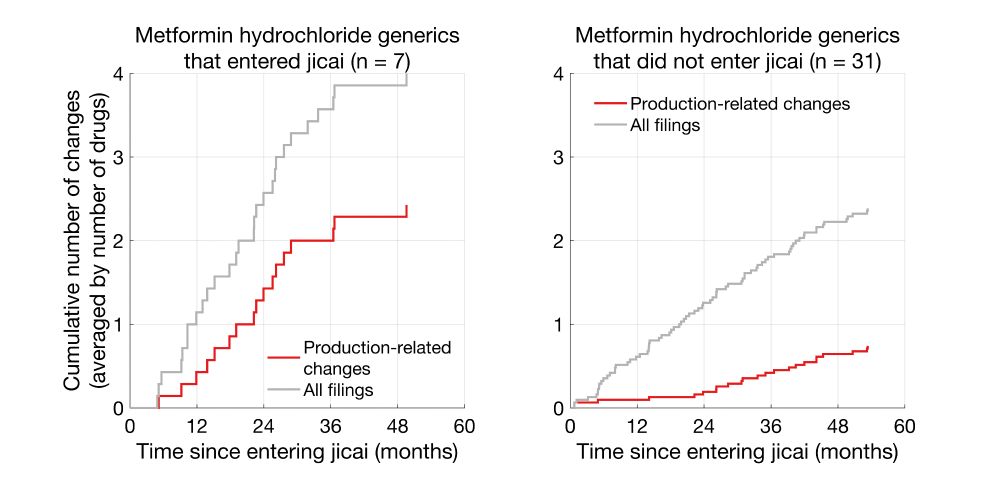
Clearly, Telmisartan generics that entered jicai underwent more production-related changes than non-jicai generics.
Same trends were also seen for metformin hydrochloride generics.
Here I plotted for each drug the date it entered jicai and dates of all supplemental filings.
* 45.7% of jicai drugs changed suppliers post-approval
* 16.4% changed production processes
* 15.3% changed manufacturing sites
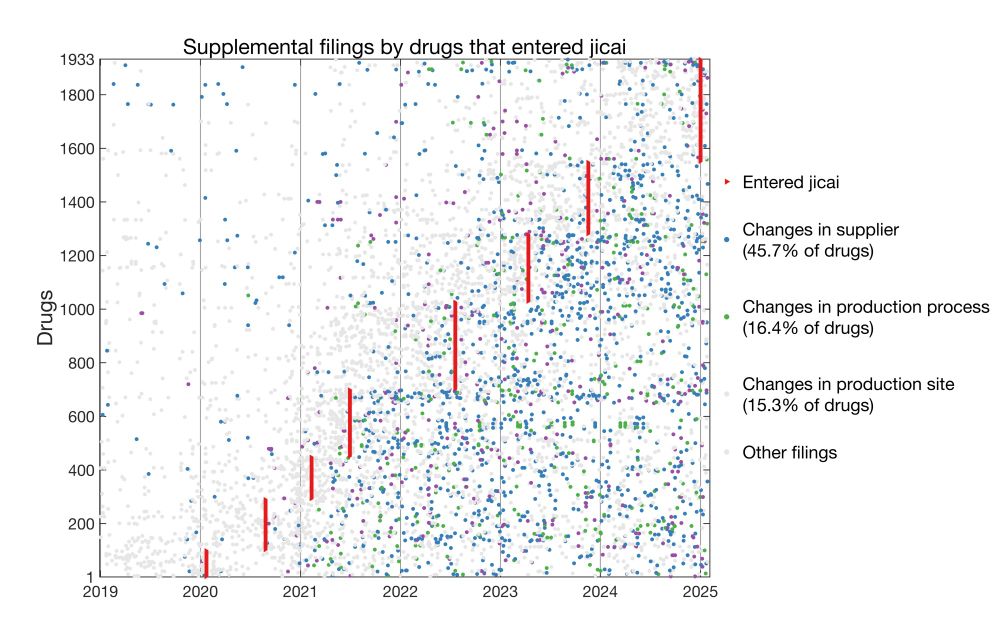
Here I plotted for each drug the date it entered jicai and dates of all supplemental filings.
* 45.7% of jicai drugs changed suppliers post-approval
* 16.4% changed production processes
* 15.3% changed manufacturing sites
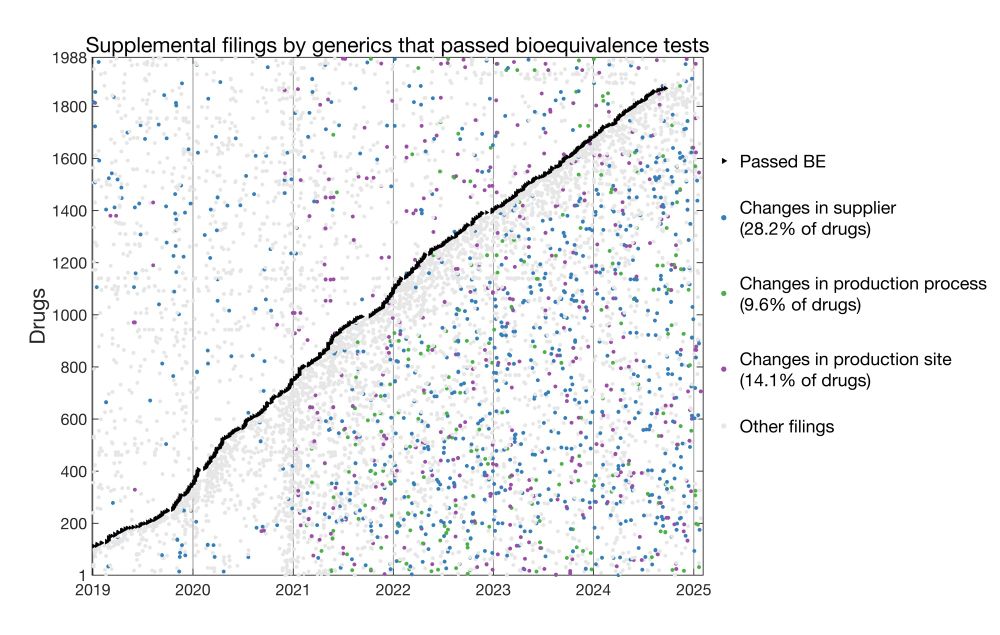
Here I plotted for each drug the date it passed BE and dates of all supplemental filings.
* 28.2% of generics changed suppliers post-approval
* 9.6% changed production processes
* 14.1% changed manufacturing sites
Here I plotted for each drug the date it passed BE and dates of all supplemental filings.
* 28.2% of generics changed suppliers post-approval
* 9.6% changed production processes
* 14.1% changed manufacturing sites
Here I quantified how prevalent these changes are.

Here I quantified how prevalent these changes are.
I analyzed >160k supplemental filings and found widespread post-approval changes in generics and jicai drugs. Importantly, jicai drugs underwent more changes than non-jicai counterparts.
bsky.app/profile/airm...



I analyzed >160k supplemental filings and found widespread post-approval changes in generics and jicai drugs. Importantly, jicai drugs underwent more changes than non-jicai counterparts.
bsky.app/profile/airm...
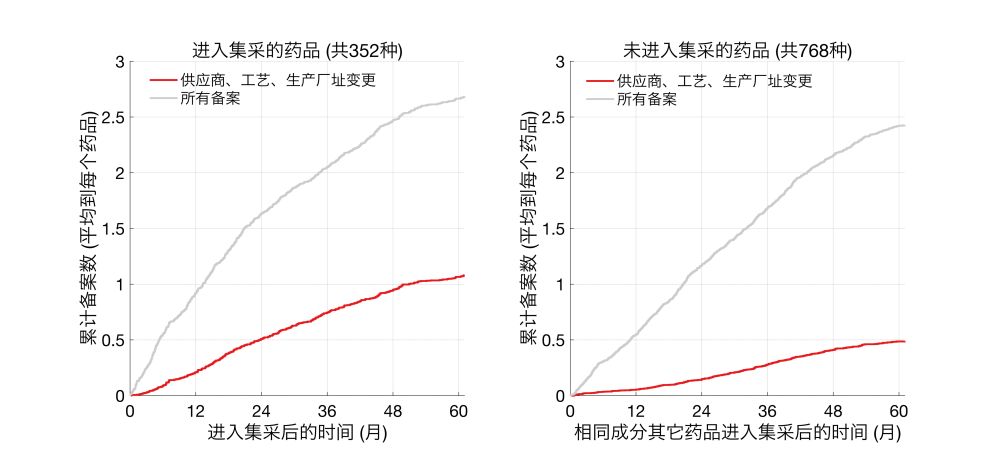
统计生产环节变更和所有备案次数后发现,进入集采的药品和未进入集采的药品,总备案数类似。然而,在关键的、可能会影响药物成分、药效的变更上,进入集采的药品发生了更多的生产环节的变更,约2倍于未进入集采的药品。
再次强调,这里的分析并非简单地对比所有进入集采的药品和未进入集采的药品。而是找到了对应的同成分的集采、非集采仿制药,进行对比。
统计生产环节变更和所有备案次数后发现,进入集采的药品和未进入集采的药品,总备案数类似。然而,在关键的、可能会影响药物成分、药效的变更上,进入集采的药品发生了更多的生产环节的变更,约2倍于未进入集采的药品。
再次强调,这里的分析并非简单地对比所有进入集采的药品和未进入集采的药品。而是找到了对应的同成分的集采、非集采仿制药,进行对比。
为分析这一问题,我对比了进入集采的仿制药和未进入集采(但也通过了一致性测评)的同种药品。
比如,CDE数据库中的仿制替米沙坦片共有28种,其中7种在2021-1批进入集采(2/8日公布)。我统计了自当日起,进入集采和未进入集采的替米沙坦片进行生产环节变更(供应商、工艺、厂址)的次数,以及所有备案的次数。
我将这些次数平均到了每个药品上(集采7种,非集采21种),以便比较。进入集采的替米沙坦片相比未进入集采的,进行了更多的生产环节变更,整体备案数也更多。盐酸二甲双胍片也存在类似的现象。


为分析这一问题,我对比了进入集采的仿制药和未进入集采(但也通过了一致性测评)的同种药品。
比如,CDE数据库中的仿制替米沙坦片共有28种,其中7种在2021-1批进入集采(2/8日公布)。我统计了自当日起,进入集采和未进入集采的替米沙坦片进行生产环节变更(供应商、工艺、厂址)的次数,以及所有备案的次数。
我将这些次数平均到了每个药品上(集采7种,非集采21种),以便比较。进入集采的替米沙坦片相比未进入集采的,进行了更多的生产环节变更,整体备案数也更多。盐酸二甲双胍片也存在类似的现象。
这里我标注了每个药品进入国家集采的时间(共8批)和进行变更备案的时间。
45.7%、16.4%、15.3%的集采药品在进入集采之后,分别进行了供应商变更、生产工艺变更、生产厂址变更。三个比例都比通过一致性评价的药品更高(进入集采的仿制药需经过一致性评价,但不是所有过评的仿制药都能够进入集采)。
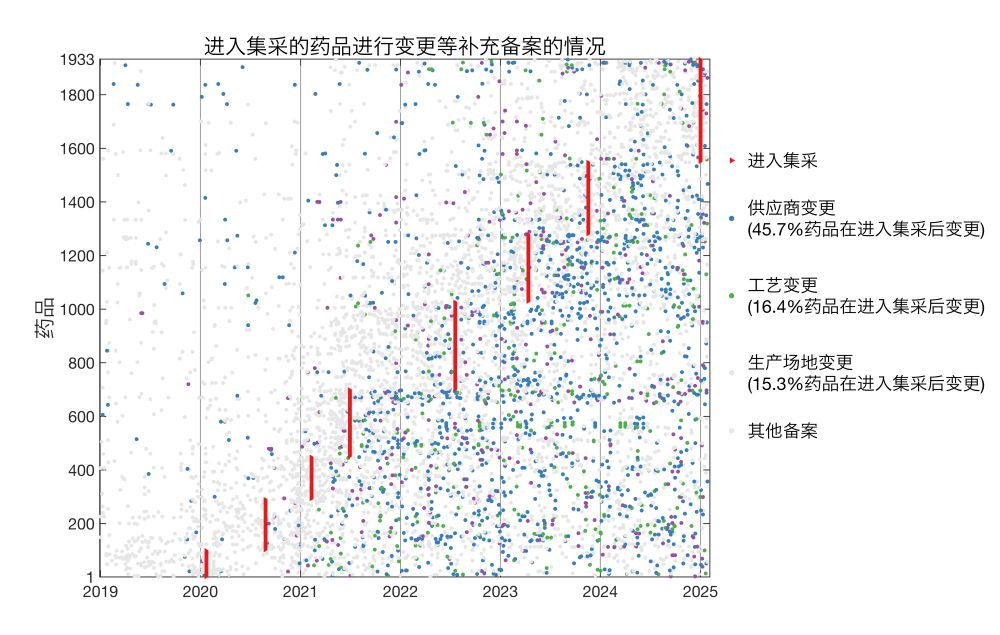
这里我标注了每个药品进入国家集采的时间(共8批)和进行变更备案的时间。
45.7%、16.4%、15.3%的集采药品在进入集采之后,分别进行了供应商变更、生产工艺变更、生产厂址变更。三个比例都比通过一致性评价的药品更高(进入集采的仿制药需经过一致性评价,但不是所有过评的仿制药都能够进入集采)。
这里,每一行代表了一种过评仿制药(共1988种),横轴代表事件发生的时间。
我标注了几种事件:过评的时间、发生各类变更的时间、进行其它备案的时间。可见,在过评后(即斜黑线右侧),出现许多例生产环节上的变更。
我对此进行了分析,只统计了过评之后的生产变更,发现28.2%、9.6%、14.1%的药品在过评后分别进行了供应商变更、生产工艺变更、生产厂址变更。
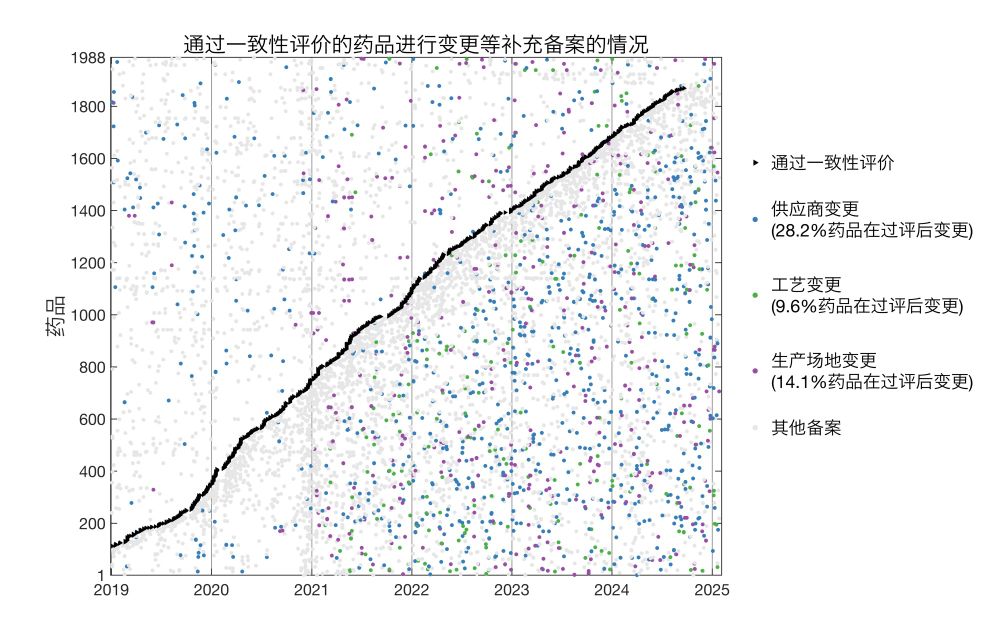
这里,每一行代表了一种过评仿制药(共1988种),横轴代表事件发生的时间。
我标注了几种事件:过评的时间、发生各类变更的时间、进行其它备案的时间。可见,在过评后(即斜黑线右侧),出现许多例生产环节上的变更。
我对此进行了分析,只统计了过评之后的生产变更,发现28.2%、9.6%、14.1%的药品在过评后分别进行了供应商变更、生产工艺变更、生产厂址变更。
经查询可见,许多药品会进行原料供应商、生产工艺、生产厂址的多项变更,并只需在省级药监部分进行备案。即使是进行了一致性评价的仿制药,进行此类变更后,也无需重新进行一致性评价。


经查询可见,许多药品会进行原料供应商、生产工艺、生产厂址的多项变更,并只需在省级药监部分进行备案。即使是进行了一致性评价的仿制药,进行此类变更后,也无需重新进行一致性评价。
实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。



实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。
Now how did the duplication happen? Turns out that both trials were run by 王文萍/李晓斌 at 辽宁中医药大学 and the samples were both tested by 安徽万邦...


Now how did the duplication happen? Turns out that both trials were run by 王文萍/李晓斌 at 辽宁中医药大学 and the samples were both tested by 安徽万邦...


1) An instance of blatant data duplication in a clinical trial for an HBV drug, appearing in material published by the drugmaker/clinical trial team.


1) An instance of blatant data duplication in a clinical trial for an HBV drug, appearing in material published by the drugmaker/clinical trial team.


But there are clearly points off the diagonal, e.g. trial CYHB2150870 has large discrepancies between ratios in the bottom 3 rows. Compare columns 3 and 6.


But there are clearly points off the diagonal, e.g. trial CYHB2150870 has large discrepancies between ratios in the bottom 3 rows. Compare columns 3 and 6.



www.cde.org.cn/main/news/vi...

www.cde.org.cn/main/news/vi...
I am in EST, and 1:50AM is when I downloaded the file.


I am in EST, and 1:50AM is when I downloaded the file.

