
一点浩然气 千里快哉风
airmovingdevice@protonmail.com
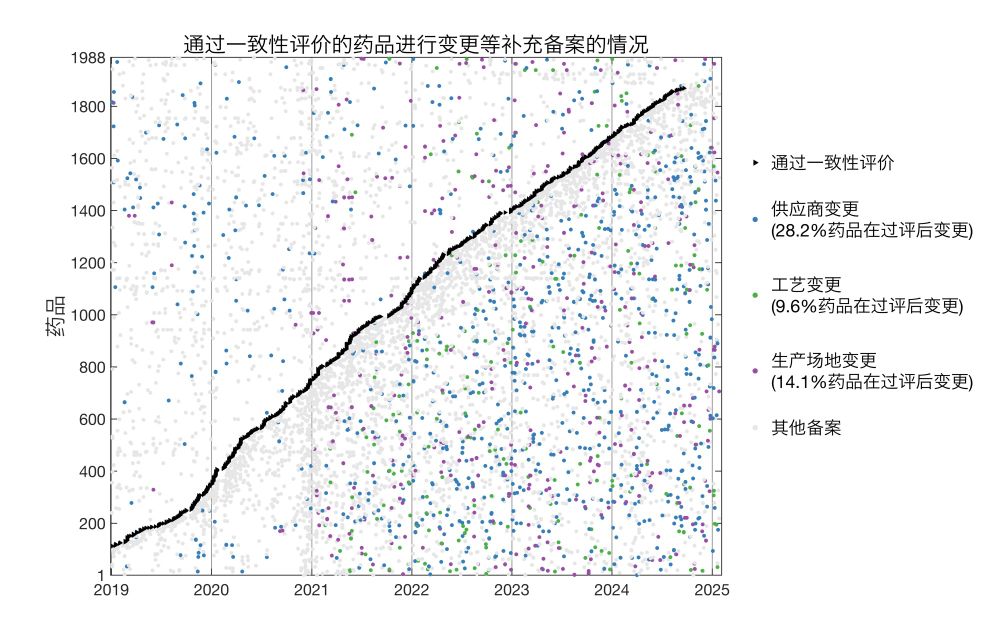
实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
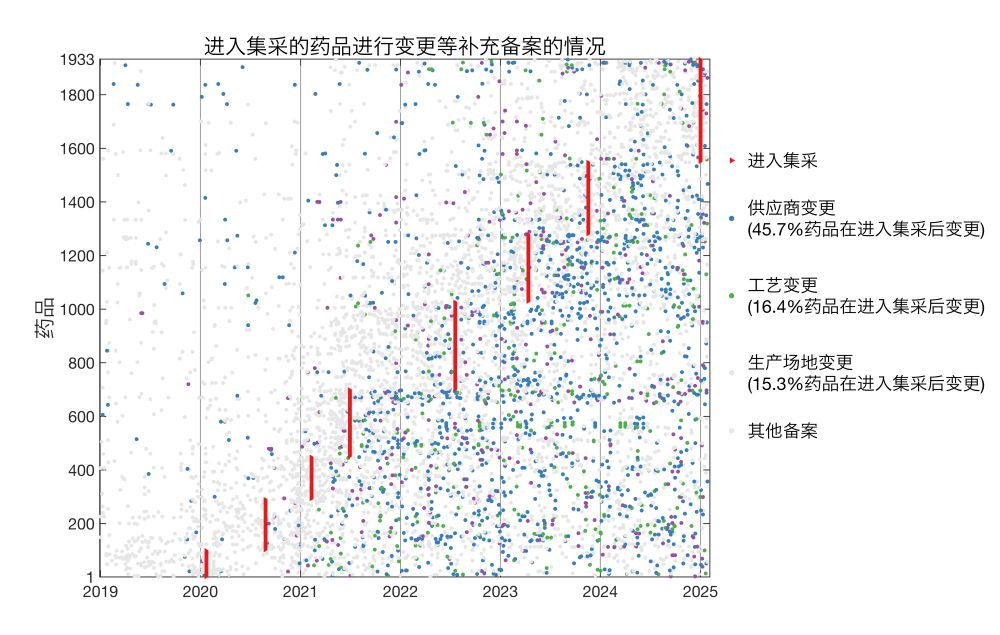
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
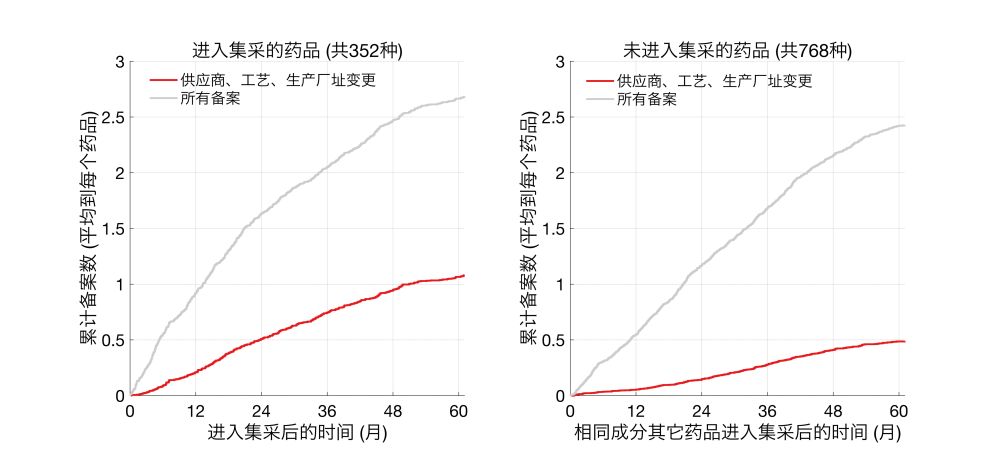
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。
这两项规定明显弱于之前征求意见稿中的硬性规定:首个中选周期内不得进行重要生产环节的变更,否则取消中选资格。
大概是多方博弈的结果,baby steps也好吧。


这两项规定明显弱于之前征求意见稿中的硬性规定:首个中选周期内不得进行重要生产环节的变更,否则取消中选资格。
大概是多方博弈的结果,baby steps也好吧。
Among cancellations with election data available, 92.9% and 86.1% cancelled grants and contracts went to Harris counties, representing 96.6% and 92.4% of total dollar amounts.
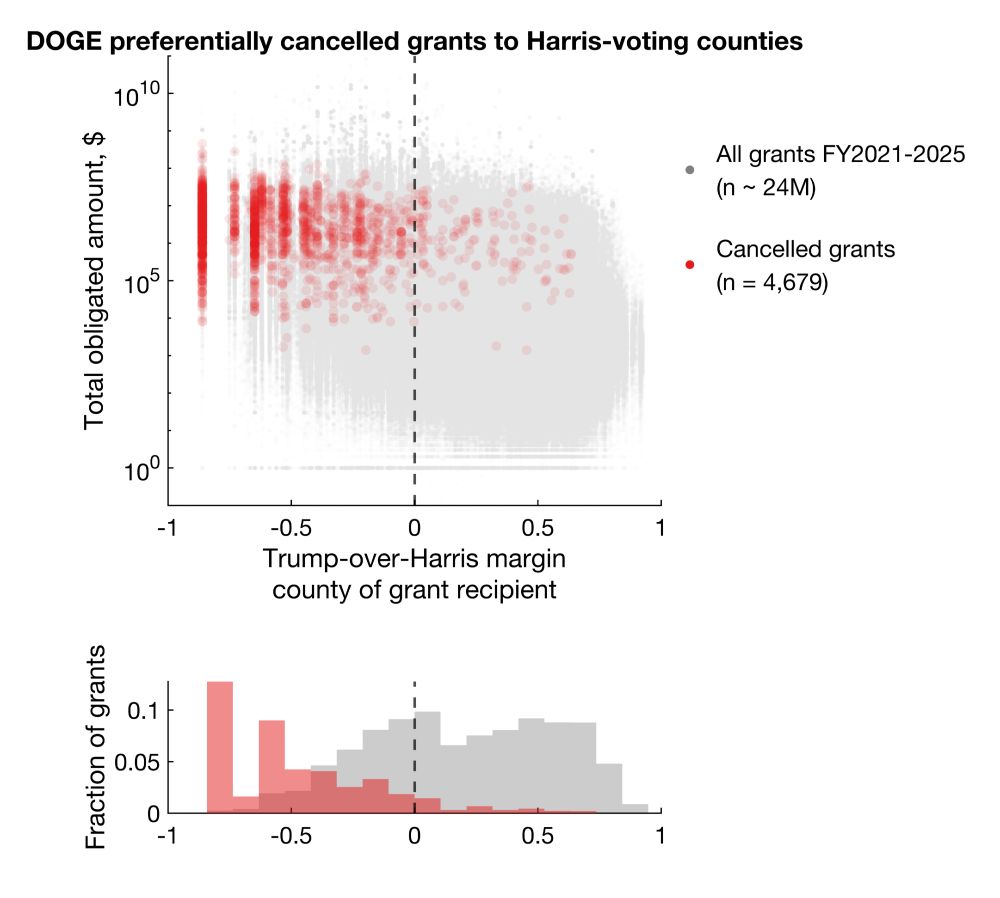
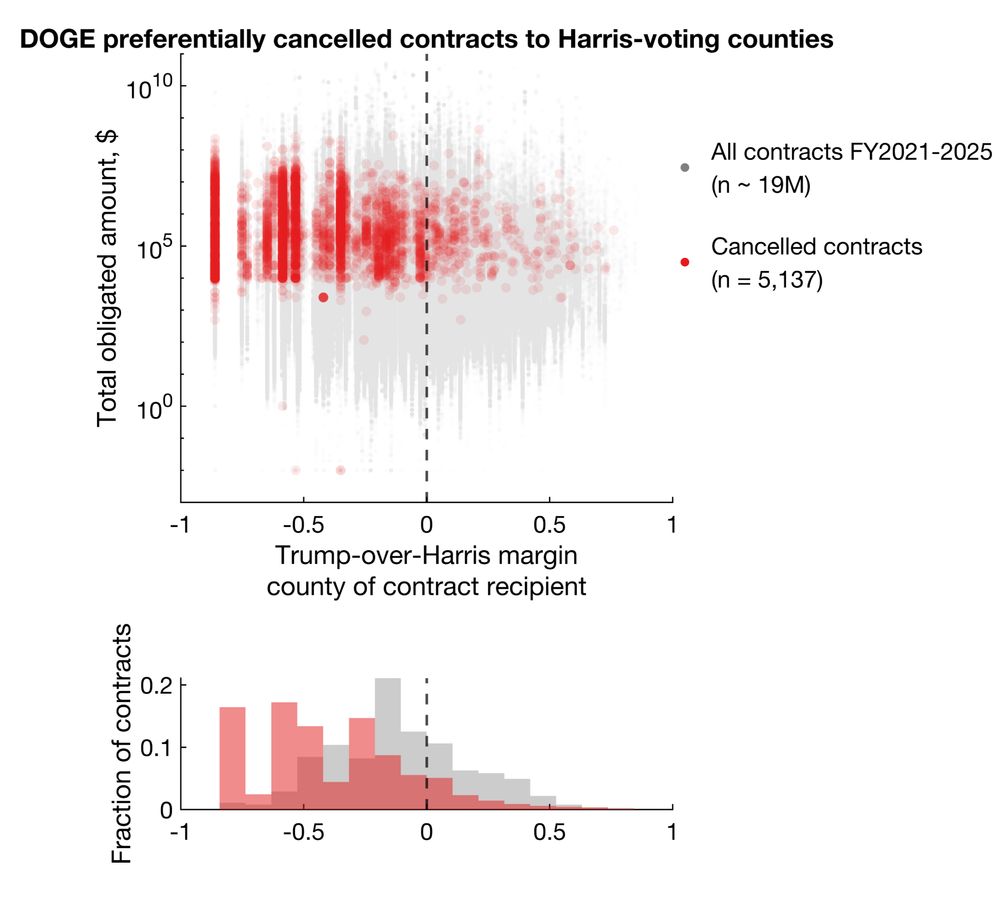
Among cancellations with election data available, 92.9% and 86.1% cancelled grants and contracts went to Harris counties, representing 96.6% and 92.4% of total dollar amounts.
I analyzed >160k supplemental filings and found widespread post-approval changes in generics and jicai drugs. Importantly, jicai drugs underwent more changes than non-jicai counterparts.
bsky.app/profile/airm...
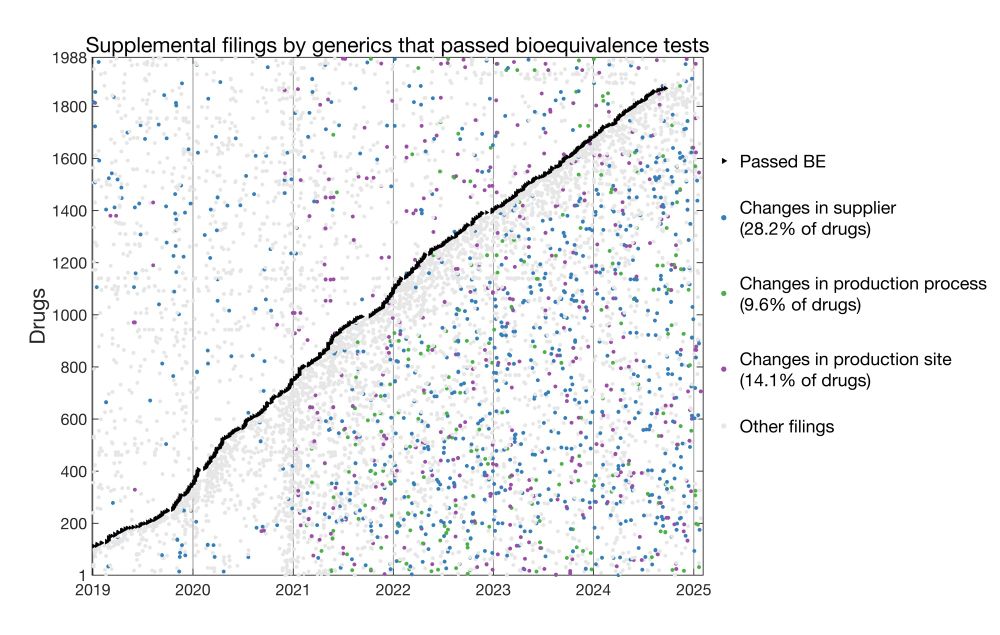
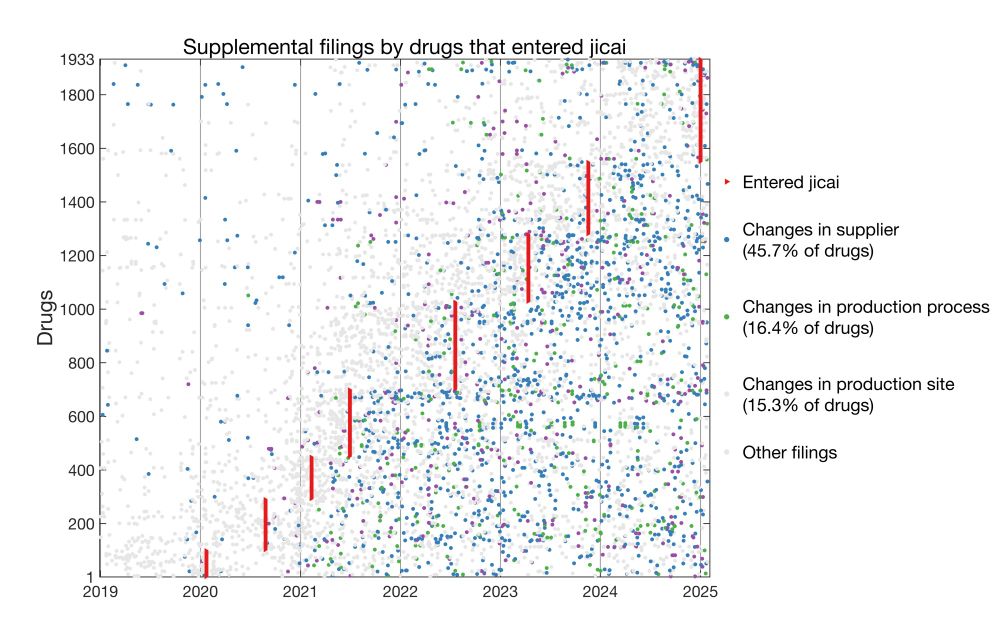
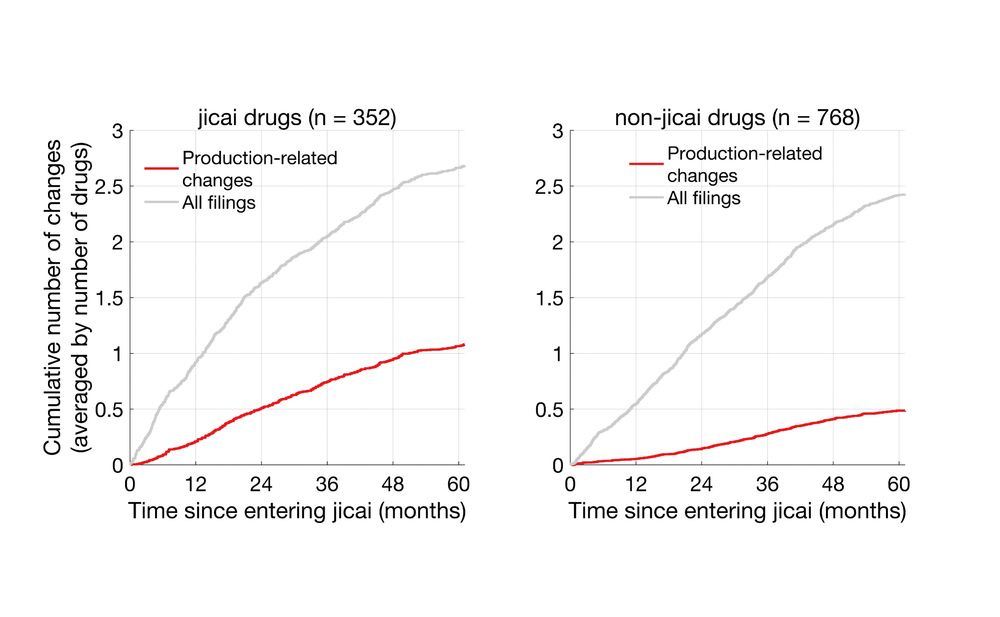
I analyzed >160k supplemental filings and found widespread post-approval changes in generics and jicai drugs. Importantly, jicai drugs underwent more changes than non-jicai counterparts.
bsky.app/profile/airm...
实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。



实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。
实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。



实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。
A small part of me wants some credit, but it’s more the powerlessness that I can’t explain and defend my data on my own terms.
A small part of me wants some credit, but it’s more the powerlessness that I can’t explain and defend my data on my own terms.
I believe they cannot be attributed to simple "editing errors" as claimed by NMPA.
This points to potential fraud, negligence, and poor NMPA oversight.
1) An instance of blatant data duplication in a clinical trial for an HBV drug, appearing in material published by the drugmaker/clinical trial team.


I believe they cannot be attributed to simple "editing errors" as claimed by NMPA.
This points to potential fraud, negligence, and poor NMPA oversight.
1) An instance of blatant data duplication in a clinical trial for an HBV drug, appearing in material published by the drugmaker/clinical trial team.


1) An instance of blatant data duplication in a clinical trial for an HBV drug, appearing in material published by the drugmaker/clinical trial team.
While NMPA works on their internal audit, I found more instances of data discrepancies that are hard to attribute to "editorial errors", as NMPA claimed in response to my previous posts. This gets technical but bear with me.
While NMPA works on their internal audit, I found more instances of data discrepancies that are hard to attribute to "editorial errors", as NMPA claimed in response to my previous posts. This gets technical but bear with me.
但很明显的问题经过层层审核未被发现,至少说明药监局对数据的分析核查能力不足。加上之前的低级“编辑错误”,药监局未能对一致性评价起到该有的监管作用。
While NMPA works on their internal audit, I found more instances of data discrepancies that are hard to attribute to "editorial errors", as NMPA claimed in response to my previous posts. This gets technical but bear with me.
但很明显的问题经过层层审核未被发现,至少说明药监局对数据的分析核查能力不足。加上之前的低级“编辑错误”,药监局未能对一致性评价起到该有的监管作用。
While NMPA works on their internal audit, I found more instances of data discrepancies that are hard to attribute to "editorial errors", as NMPA claimed in response to my previous posts. This gets technical but bear with me.
While NMPA works on their internal audit, I found more instances of data discrepancies that are hard to attribute to "editorial errors", as NMPA claimed in response to my previous posts. This gets technical but bear with me.
I do think that some of these data discrepancies could be honest editorial mistakes as NMPA claimed, especially ones where the entire result section was lifted from another trial.
But…
I've seen limited coverage outside China of the recent controversy over 集采 — centralized procurement of drugs, especially generics. It is a hugely important issue close to my heart.
I do think that some of these data discrepancies could be honest editorial mistakes as NMPA claimed, especially ones where the entire result section was lifted from another trial.
But…
www.cde.org.cn/main/news/vi...

www.cde.org.cn/main/news/vi...

I've seen limited coverage outside China of the recent controversy over 集采 — centralized procurement of drugs, especially generics. It is a hugely important issue close to my heart.
What strikes me most is how blatant and dumb the fraud is — simple copy-and-paste plagiarism of a competitor drug’s trial.
I've seen limited coverage outside China of the recent controversy over 集采 — centralized procurement of drugs, especially generics. It is a hugely important issue close to my heart.
What strikes me most is how blatant and dumb the fraud is — simple copy-and-paste plagiarism of a competitor drug’s trial.
I've seen limited coverage outside China of the recent controversy over 集采 — centralized procurement of drugs, especially generics. It is a hugely important issue close to my heart.
I've seen limited coverage outside China of the recent controversy over 集采 — centralized procurement of drugs, especially generics. It is a hugely important issue close to my heart.

