
@RKMRC_NDP @mcautonomous Alumnus; Bibliophile 📚; Orophile🗻; Caeruleaphile 💙; Shutterbug 📸; Amateur Badminton Player 🏸.
@cnrsbiologie.bsky.social
@univ-amu.fr
@cea.fr
@frenchbic.bsky.social pubs.acs.org/doi/10.1021/...

@cnrsbiologie.bsky.social
@univ-amu.fr
@cea.fr
@frenchbic.bsky.social pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/full/10....

pubs.acs.org/doi/full/10....
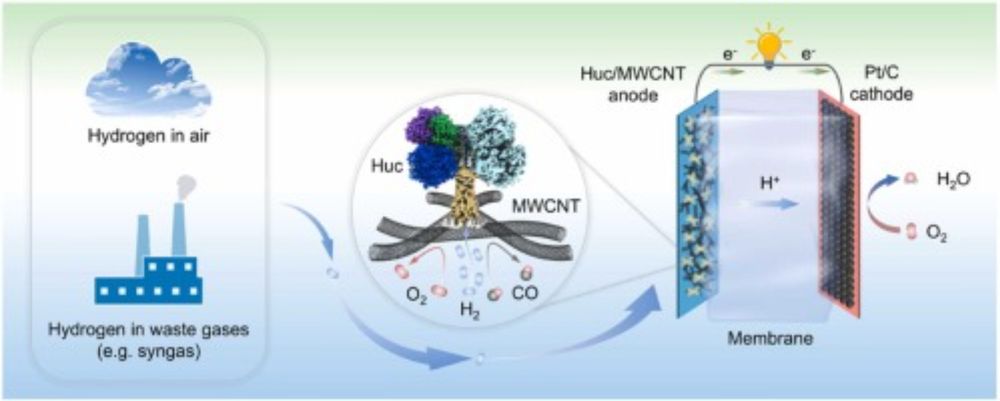

doi.org/10.1021/jacs...

doi.org/10.1021/jacs...
doi.org/10.1021/jacs...

doi.org/10.1021/jacs...

Unraveling Photo-Driven H2 Production with a Bio-Inspired NiFe Complex: Revealing an Unexpected Hydrogen Atom Source | Journal of the American Chemical Society pubs.acs.org/doi/10.1021/...
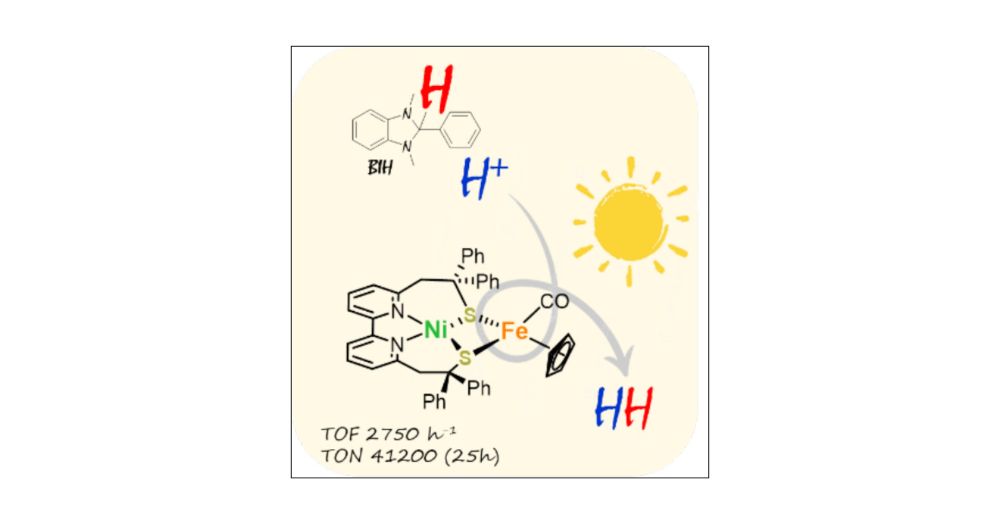
Unraveling Photo-Driven H2 Production with a Bio-Inspired NiFe Complex: Revealing an Unexpected Hydrogen Atom Source | Journal of the American Chemical Society pubs.acs.org/doi/10.1021/...

