
Keen to explore cutting-edge laboratory experiments and sharpen your experimental skills? We’re looking for applications for a PhD position in physical chemistry of the atmosphere.
+41 56 310 43 01 | thorsten.bartels-rausch@psi.ch

Keen to explore cutting-edge laboratory experiments and sharpen your experimental skills? We’re looking for applications for a PhD position in physical chemistry of the atmosphere.
+41 56 310 43 01 | thorsten.bartels-rausch@psi.ch


Here we are, opening the Faraday Discussion on Atmospheric Science in the Cold in London. 👩🏼💻👨🏽💻👨🔬🧑🏻🔬👩🏼🔬👨🏼🔧🧑🏻🔧😎🥳🧐


Here we are, opening the Faraday Discussion on Atmospheric Science in the Cold in London. 👩🏼💻👨🏽💻👨🔬🧑🏻🔬👩🏼🔬👨🏼🔧🧑🏻🔧😎🥳🧐

"Thinking time is often undervalued; it is rarely, if ever, quantified in employment practices."
www.nature.com/articles/d41...

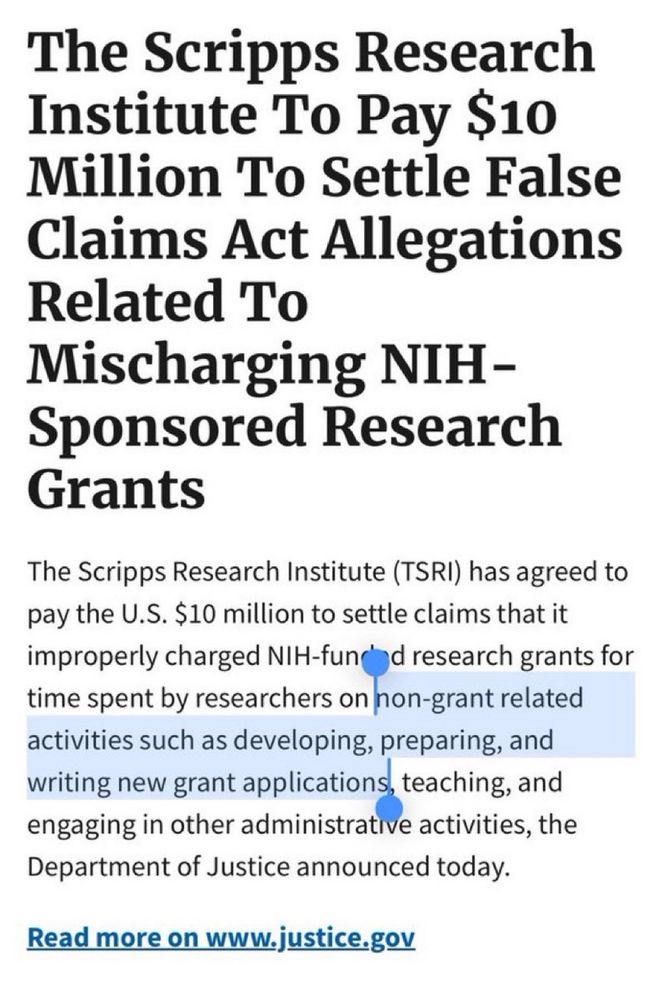
"Thinking time is often undervalued; it is rarely, if ever, quantified in employment practices."
www.nature.com/articles/d41...

+ Report: arctic.noaa.gov/report-card/...
+ Video: www.youtube.com/watch?v=0yqG...
+ Summary: arctic.noaa.gov/report-card/...
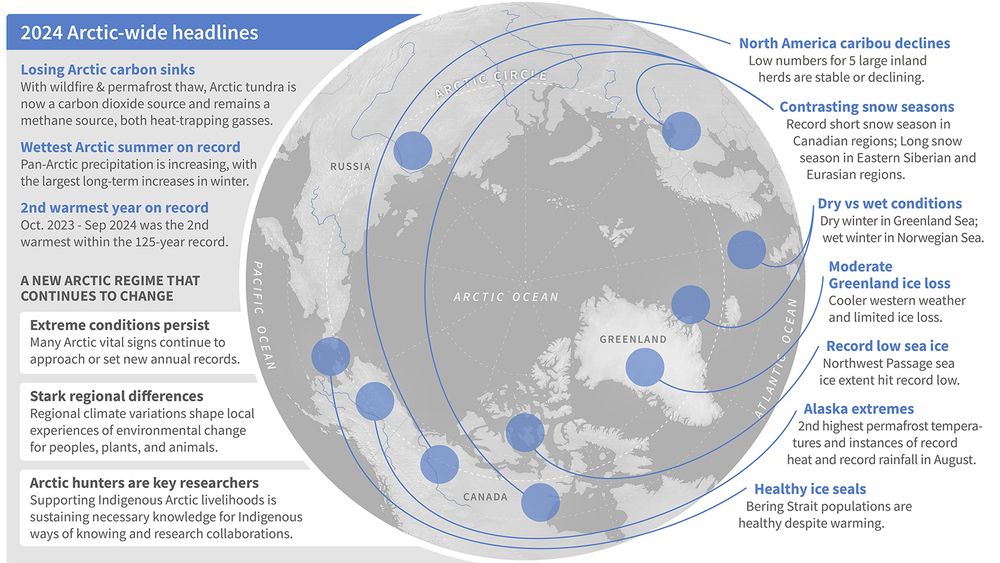
+ Report: arctic.noaa.gov/report-card/...
+ Video: www.youtube.com/watch?v=0yqG...
+ Summary: arctic.noaa.gov/report-card/...








