
#biotech #biosky #ASGCT2025
www.biopharmadive.com/news/crispr-...
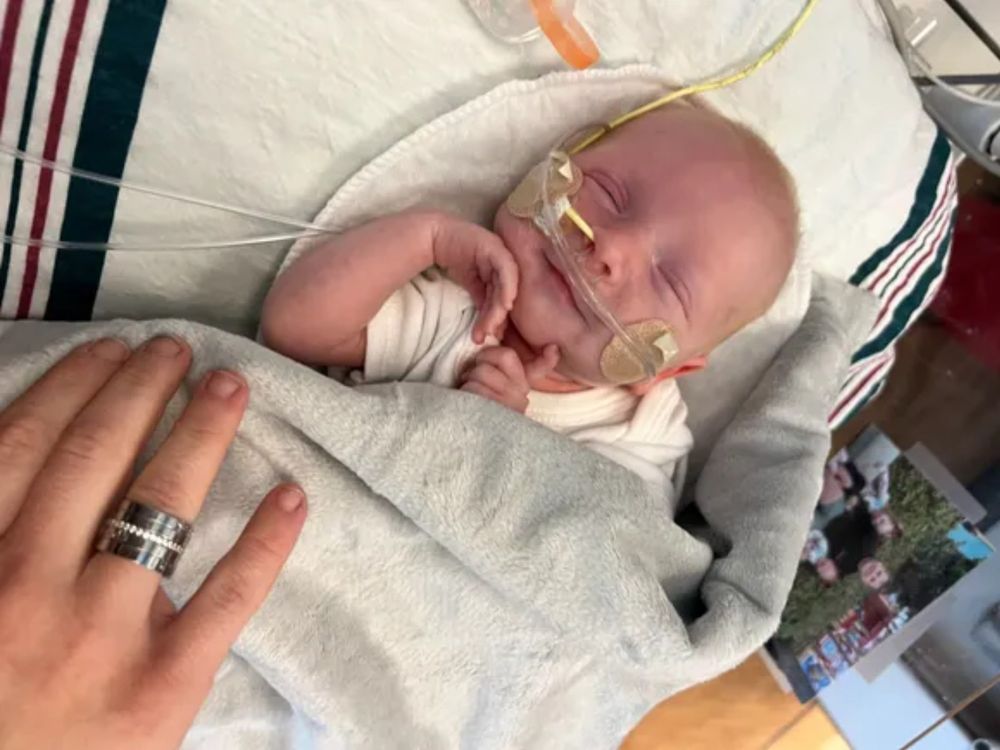
#biotech #biosky #ASGCT2025
www.biopharmadive.com/news/crispr-...

www.biopharmadive.com/news/peter-m...

www.biopharmadive.com/news/peter-m...
The OK is a milestone for research into the rare disease, which has frustrated drugmakers' prior attempts. More from @benthefidler.bsky.social here:
www.biopharmadive.com/news/soleno-...

The OK is a milestone for research into the rare disease, which has frustrated drugmakers' prior attempts. More from @benthefidler.bsky.social here:
www.biopharmadive.com/news/soleno-...
The new indication is seen as vital for Alnylam's growth, as @benthefidler.bsky.social reports:
www.biopharmadive.com/news/alnylam...

The new indication is seen as vital for Alnylam's growth, as @benthefidler.bsky.social reports:
www.biopharmadive.com/news/alnylam...
www.biopharmadive.com/news/vertex-...
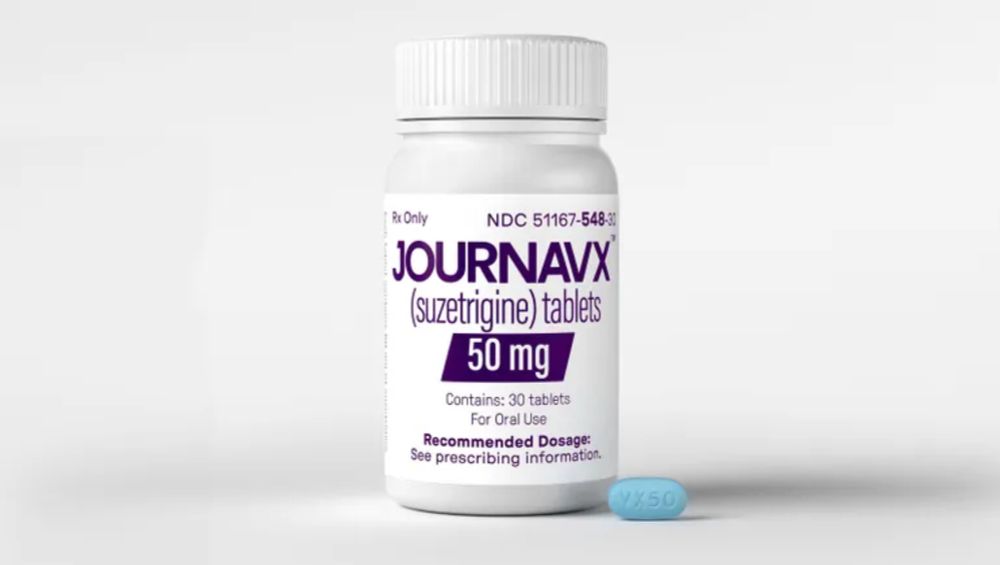
www.biopharmadive.com/news/vertex-...
www.biopharmadive.com/news/pd1-veg...
www.biopharmadive.com/news/pd1-veg...

