
@eddiecliff.bsky.social
oncodaily.com/voices/lymph...
#OncoDaily #Oncology #Cancer #Health #Medicine #MedEd #MedOnc #MedNews

@eddiecliff.bsky.social
oncodaily.com/voices/lymph...
#OncoDaily #Oncology #Cancer #Health #Medicine #MedEd #MedOnc #MedNews
@eddiecliff.bsky.social @mskcancercenter.bsky.social
@eddiecliff.bsky.social @mskcancercenter.bsky.social
Surprisingly, head-to-head trials were as likely to be successful as add-on trials.
academic.oup.com/jnci/advance... #mmsm #myeloma

Surprisingly, head-to-head trials were as likely to be successful as add-on trials.
academic.oup.com/jnci/advance... #mmsm #myeloma
Thanks David Russler-Germain + collabs
aacrjournals.org/clincancerre...

Thanks David Russler-Germain + collabs
aacrjournals.org/clincancerre...
ja.ma/3Kz6WnV

ja.ma/3Kz6WnV
- 740 pts, 59% R/R
- R/R: ORR 60% non-GC (vs 36% GC), PFS better in non-GC
- 1L: no diff in outcomes
Hans useful in choosing pola in R/R. In 1L, supports POLARIX that pola mitigates worse non-GC outcomes. #lymsm
aacrjournals.org/clincancerre...


- 740 pts, 59% R/R
- R/R: ORR 60% non-GC (vs 36% GC), PFS better in non-GC
- 1L: no diff in outcomes
Hans useful in choosing pola in R/R. In 1L, supports POLARIX that pola mitigates worse non-GC outcomes. #lymsm
aacrjournals.org/clincancerre...
🔗 jamanetwork.com/journals/jam...
🔗 jamanetwork.com/journals/jam...
ja.ma/4lXJcYs

ja.ma/4lXJcYs
12/50 vaccines
10 nonmalignant haem
11 neurology
5 nonvaccine ID
>60% conversions to regular approval based on surrogate endpoints
jamanetwork.com/journals/jam... @portalresearch.org @akesselheim.bsky.social

12/50 vaccines
10 nonmalignant haem
11 neurology
5 nonvaccine ID
>60% conversions to regular approval based on surrogate endpoints
jamanetwork.com/journals/jam... @portalresearch.org @akesselheim.bsky.social
https://ja.ma/4lRpuge

https://ja.ma/4lRpuge


Predictors of withdrawal of anticancer drug indications granted accelerated approval: a retrospective cohort study
Read the whole paper here: www.thelancet.com/journals/ecl...
#TheLancet #Oncology #OncologyResearch #MedSky
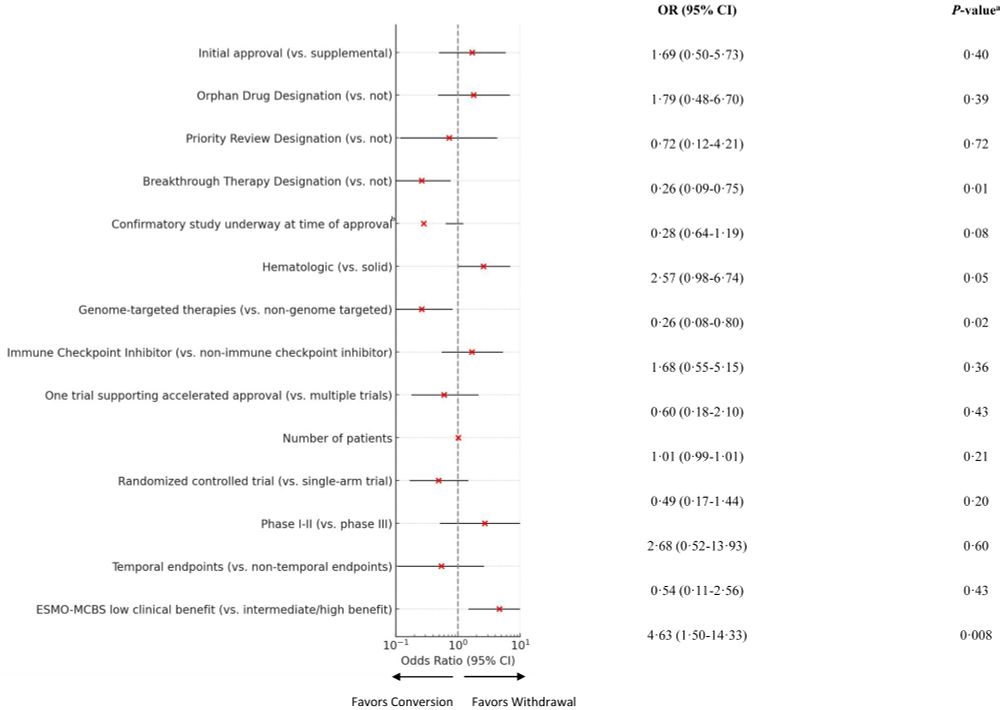
Predictors of withdrawal of anticancer drug indications granted accelerated approval: a retrospective cohort study
Read the whole paper here: www.thelancet.com/journals/ecl...
#TheLancet #Oncology #OncologyResearch #MedSky
We review advances in measuring & reporting newly-described AEs and provide analytic & AE visualization tools for dissemination.
buff.ly/SchRHhq
Thread ⬇️🧵


We review advances in measuring & reporting newly-described AEs and provide analytic & AE visualization tools for dissemination.
buff.ly/SchRHhq
Thread ⬇️🧵
👇 check out this figure from our @thelancethaem.bsky.social adverse event commission
www.thelancet.com/journals/lan... @broeckelmann.bsky.social

👇 check out this figure from our @thelancethaem.bsky.social adverse event commission
www.thelancet.com/journals/lan... @broeckelmann.bsky.social


>70% of non-relapse deaths are due to infection.
Need to prioritise measurement, reporting, prophylaxis, early intervention.


>70% of non-relapse deaths are due to infection.
Need to prioritise measurement, reporting, prophylaxis, early intervention.
Led by Dr Hwang we show when oncology drugs withdrawn due to negative trials are still accessible off-abel, prescribing plummets anyway
Romidepsin a notable exception, unsurprising to lymphoma docs who use it in TFH lymphomas
jamanetwork.com/journals/jam... @portalresearch.org

Led by Dr Hwang we show when oncology drugs withdrawn due to negative trials are still accessible off-abel, prescribing plummets anyway
Romidepsin a notable exception, unsurprising to lymphoma docs who use it in TFH lymphomas
jamanetwork.com/journals/jam... @portalresearch.org
podcasts.apple.com/us/podcast/b...
Check out Blood Cancer Talks on your favourite podcast platform. #leusm #leukemia #cml

podcasts.apple.com/us/podcast/b...
Check out Blood Cancer Talks on your favourite podcast platform. #leusm #leukemia #cml

75% 3-year progression free survival
ashpublications.org/blood/articl...
Randomised ZUMA-23 data eagerly awaited
#lymsm #lymphoma

75% 3-year progression free survival
ashpublications.org/blood/articl...
Randomised ZUMA-23 data eagerly awaited
#lymsm #lymphoma
Tricky disease to treat. Perhaps combo of chemo and bispecific might offer better long term control
ashpublications.org/blood/articl... #lymsm #lymphoma

Tricky disease to treat. Perhaps combo of chemo and bispecific might offer better long term control
ashpublications.org/blood/articl... #lymsm #lymphoma
www.sciencedirect.com/science/arti... #lymphoma #lymsm
www.sciencedirect.com/science/arti... #lymphoma #lymsm



