
@gerri-sefton.bksy.social
@profberniecarter.bsky.social
@ejlim.bsky.social and others not on bsky.
@gerri-sefton.bksy.social
@profberniecarter.bsky.social
@ejlim.bsky.social and others not on bsky.
1/ Latest paper from DETECT study led by Abbey Bracken

1/ Latest paper from DETECT study led by Abbey Bracken
@resprofnews.bsky.social
www.researchprofessionalnews.com/rr-news-uk-v...

@resprofnews.bsky.social
www.researchprofessionalnews.com/rr-news-uk-v...
We’re the Royal College of Paediatrics and Child Health. We represent over 24,000 paediatricians in the UK and internationally.
Follow us to hear about our work to support #Paediatricians and transform #ChildHealth.

We’re the Royal College of Paediatrics and Child Health. We represent over 24,000 paediatricians in the UK and internationally.
Follow us to hear about our work to support #Paediatricians and transform #ChildHealth.
2/ The BATCH trial was a pragmatic, multicentre, open-label, individually randomised, controlled trial conducted in 15 hospitals in England and Wales between June 2018, and Oct 2022.
www.nature.com/articles/s41...

www.nature.com/articles/s41...
Does not appear helpful in reducing time on IV Abx in this setting.
#IDSky #AMR #AMSsky #pediatrics
www.sciencedirect.com/science/arti...

Does not appear helpful in reducing time on IV Abx in this setting.
#IDSky #AMR #AMSsky #pediatrics
www.sciencedirect.com/science/arti...
news.liverpool.ac.uk/2025/01/08/p...
@nihr.bsky.social @livunihls.bsky.social @livuni-ives.bsky.social

news.liverpool.ac.uk/2025/01/08/p...
@nihr.bsky.social @livunihls.bsky.social @livuni-ives.bsky.social
👉In 1 year, 4% of deaths were AMR-attributed & NONE were recorded on death certificates!👈
Need to quantify this better to increase awareness! #IDSky @jac-amr.bsky.social
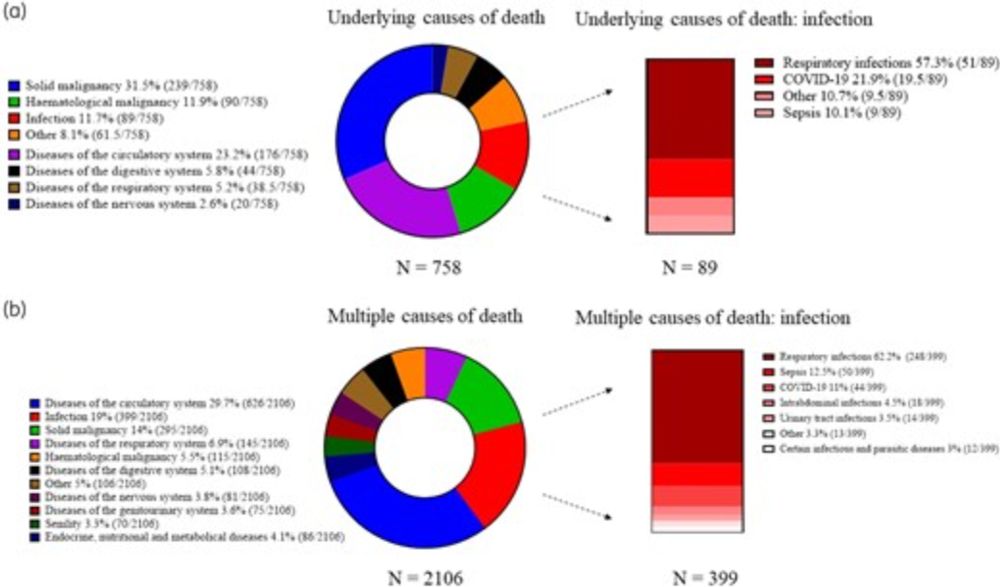
👉In 1 year, 4% of deaths were AMR-attributed & NONE were recorded on death certificates!👈
Need to quantify this better to increase awareness! #IDSky @jac-amr.bsky.social
16/ Huge thanks to all the team including @emmatj.bsky.social, @ceumateus.bsky.social @cawaldrom.bsky.social @kerryhood.bsky.social @saulfaust.bsky.social @jpreso.bsky.social and others not on here. Funded by @nihr.bsky.social
16/ Huge thanks to all the team including @emmatj.bsky.social, @ceumateus.bsky.social @cawaldrom.bsky.social @kerryhood.bsky.social @saulfaust.bsky.social @jpreso.bsky.social and others not on here. Funded by @nihr.bsky.social
12/ A better understanding of the complex interactions influencing whether/how/ why clinicians act on test results to make antibiotic prescribing decisions will improve trial intervention fidelity and facilitate implementation and scale-up of tests shown to be effective.
12/ A better understanding of the complex interactions influencing whether/how/ why clinicians act on test results to make antibiotic prescribing decisions will improve trial intervention fidelity and facilitate implementation and scale-up of tests shown to be effective.
10/ Fourthly, and perhaps most importantly, consider the context. Most of the participants (83%) were recruited from sites with robust antimicrobial stewardship (AMS) programmes. PCT guided algorithms add little value where median duration of IV antibiotics is 100 hrs (4 days).
10/ Fourthly, and perhaps most importantly, consider the context. Most of the participants (83%) were recruited from sites with robust antimicrobial stewardship (AMS) programmes. PCT guided algorithms add little value where median duration of IV antibiotics is 100 hrs (4 days).

