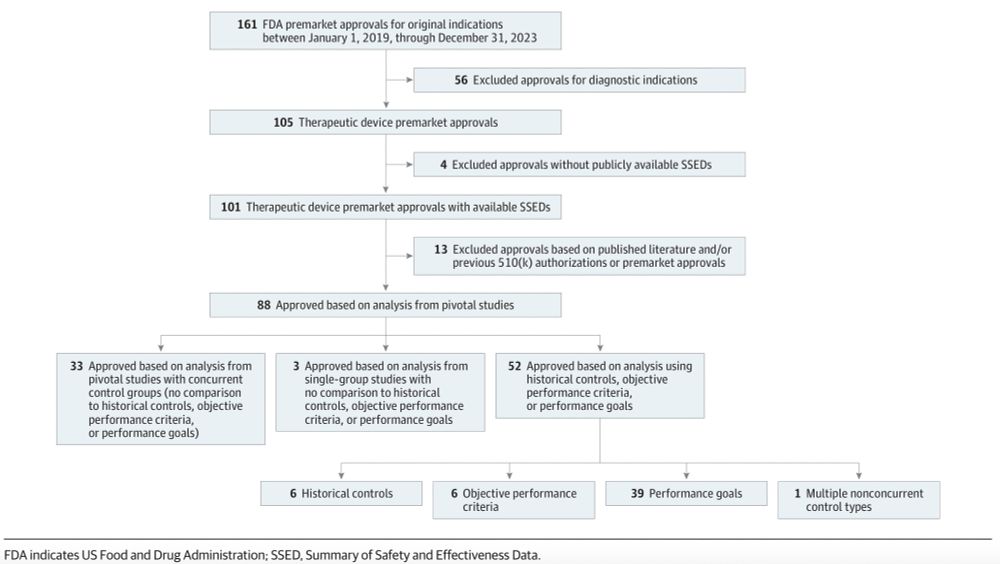
@fda.gov accelerated approval even after failed confirmatory trials? Yes—though less aggressively, finds Dr. Maryam Mooghali’s #ASCO25 Merit Award-winning research.
🔗 meetings.asco.org/abstracts-pr...
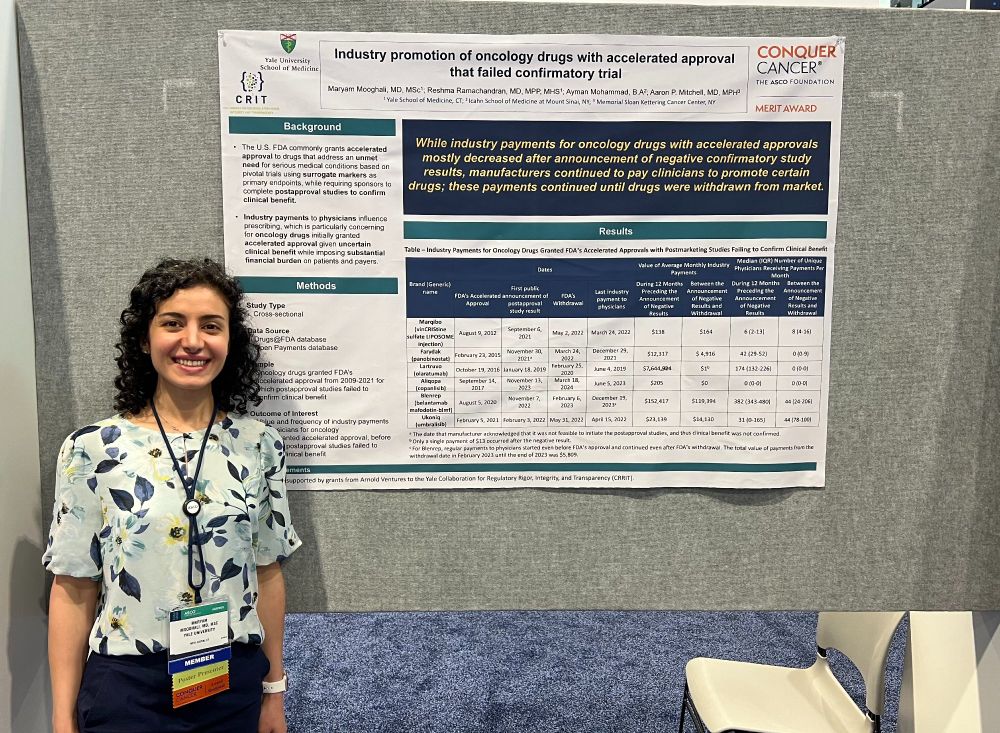
@fda.gov accelerated approval even after failed confirmatory trials? Yes—though less aggressively, finds Dr. Maryam Mooghali’s #ASCO25 Merit Award-winning research.
🔗 meetings.asco.org/abstracts-pr...
@jsross119.bsky.social @genpatient.bsky.social
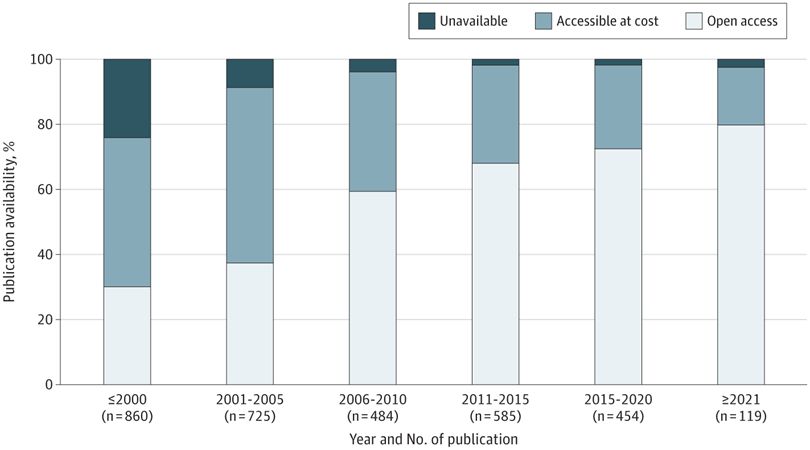
@jsross119.bsky.social @genpatient.bsky.social




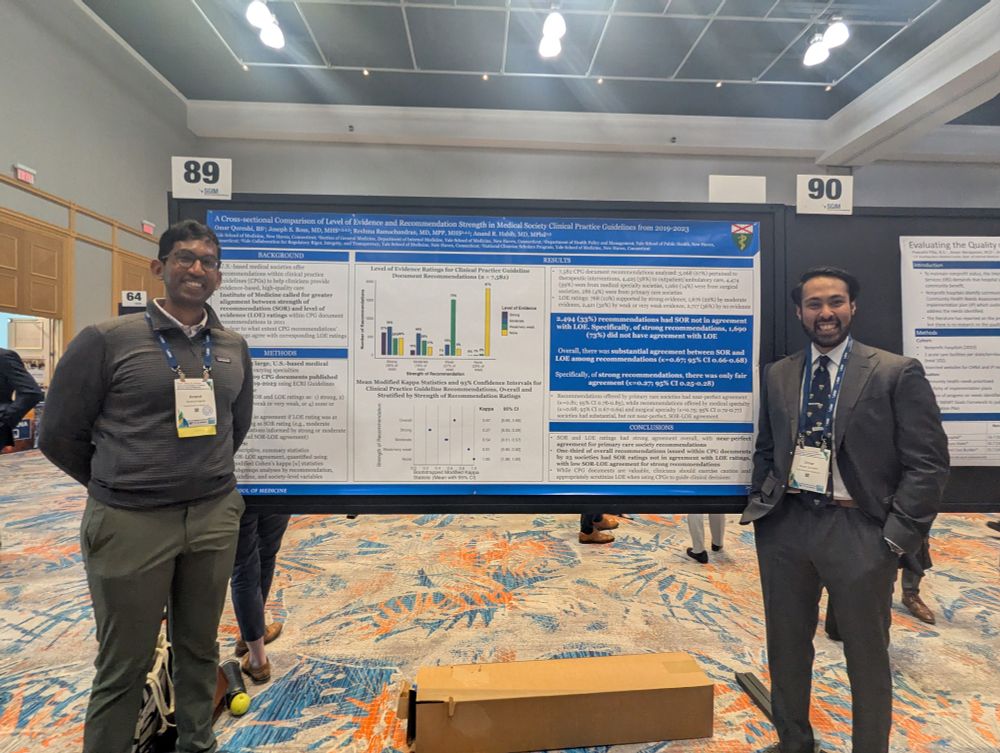



www.regulations.gov/comment/FDA-...

www.regulations.gov/comment/FDA-...

(Post 5/9)
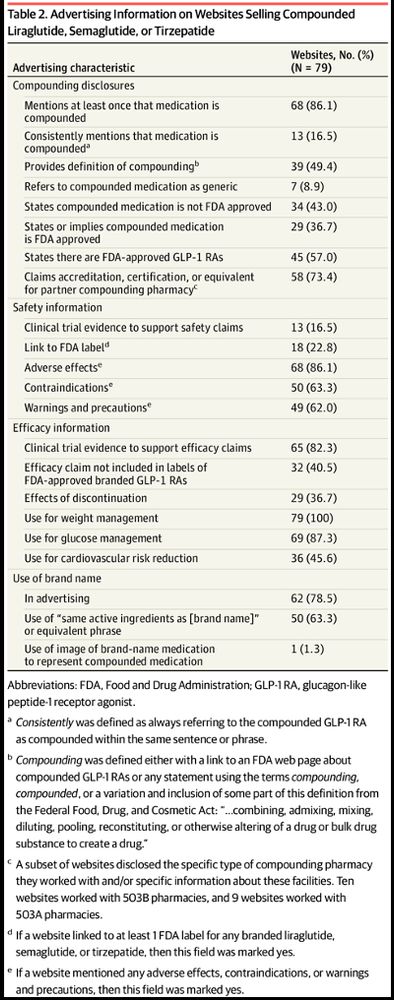
(Post 5/9)
(Post 2/9)

(Post 2/9)

www.healthaffairs.org/doi/full/10....

www.healthaffairs.org/doi/full/10....