Our new paper in ACS Catalysis discusses ORR on oriented graphitic carbon materials, their fundamental electrochemical properties and surface-structure-dependent differences in reaction mechanisms. A long and complex story, with hopefully more to come!
pubs.acs.org/doi/10.1021/...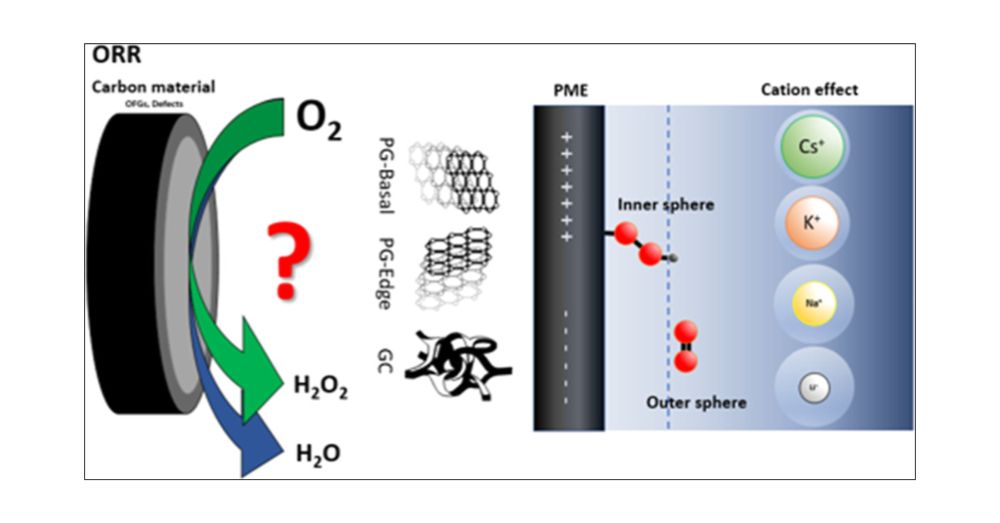
Electrochemical Insights into Hydrogen Peroxide Generation on Carbon Electrodes: Influence of Defects, Oxygen Functional Groups, and Alkali Metals in the Electrolyte
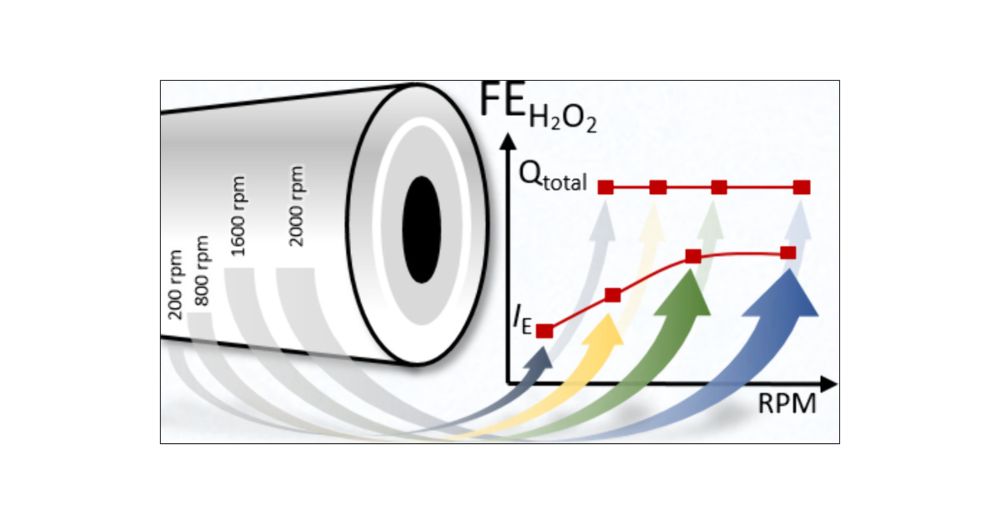
Hydrogen peroxide (H2O2) is an environmentally friendly oxidant, with production reaching 5.7 million tons by 2028 and a market size of USD 4.04 billion by 2029. Understanding the mechanism of oxygen reduction to H2O2 and the structure–activity relations on carbon materials is, therefore, of high significance for the more environmentally friendly synthesis of this important chemical. We have used oriented pyrolytic graphite (PG-edge and PG-basal) and glassy carbon (GC) as model electrodes to investigate the influence of carbon defects, oxygen-containing functional groups, and the presence of alkali metals on the activity and selectivity toward H2O2 production under acidic conditions. Electrochemical measurements, such as rotating ring disk electrode and electrochemical impedance spectroscopy, as well as in situ Raman spectroelectrochemistry indicated that PG-basal and GC electrodes preferentially form H2O2 as the product through the two-electron pathway via inner and outer sphere mechanisms, respectively. The mechanism is significantly affected by the potential of maximal entropy, which determines the position of species in the solution within the inner or outer Helmholtz plane. The influence of alkali cations (Li+, Na+, K+, and Cs+) on the oxygen reduction reaction of these model carbon electrodes was investigated. Large cations, e.g., K+ and Cs+, showed influence on the reaction intermediates and thus on the electrodes’ selectivity. The present study provides important insights and contributions to the fundamental aspects of hydrogen peroxide production in acidic conditions and further advancements in the development of metal-free carbon-based catalysts.