ORCID: https://orcid.org/0000-0002-1621-5888
Scholar: https://shorturl.at/L6MKe
After inspiring presentations, discussions, and a visit to Casa Milà in Barcelona, we're more motivated than ever to continue our journey to enhance LED technologies with chirality! 🙌


After inspiring presentations, discussions, and a visit to Casa Milà in Barcelona, we're more motivated than ever to continue our journey to enhance LED technologies with chirality! 🙌
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
#ChemSky #CompChemSky #3DP #3Dprint #3DModels 🧪
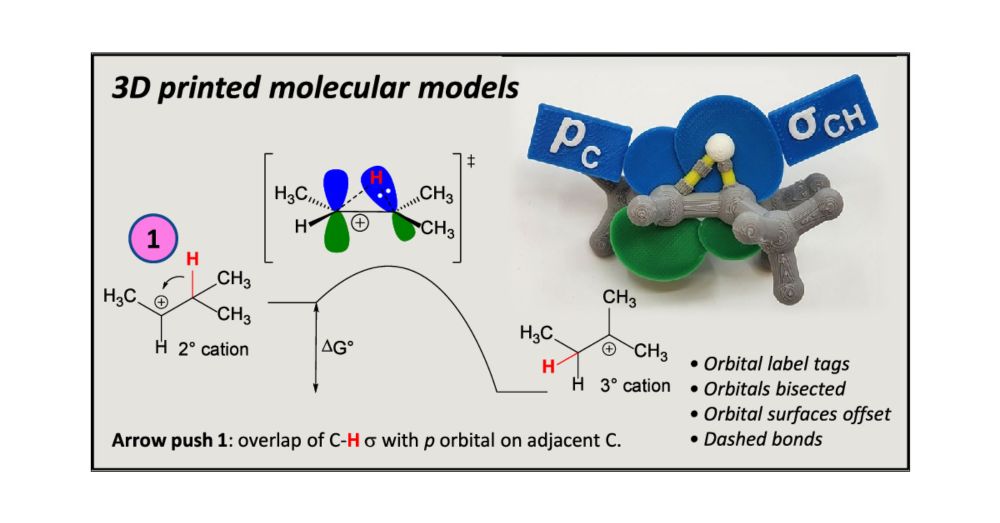
#ChemSky #CompChemSky #3DP #3Dprint #3DModels 🧪

