
Thank you Cancer Discovery (@aacrjournals.bsky.social) for the beautiful title-page graphics!

Thank you Cancer Discovery (@aacrjournals.bsky.social) for the beautiful title-page graphics!
This means reducing inflammation could unmask therapeutic vulnerabilities in aggressive clones. 6/

This means reducing inflammation could unmask therapeutic vulnerabilities in aggressive clones. 6/
But it comes at a price… 5/

But it comes at a price… 5/


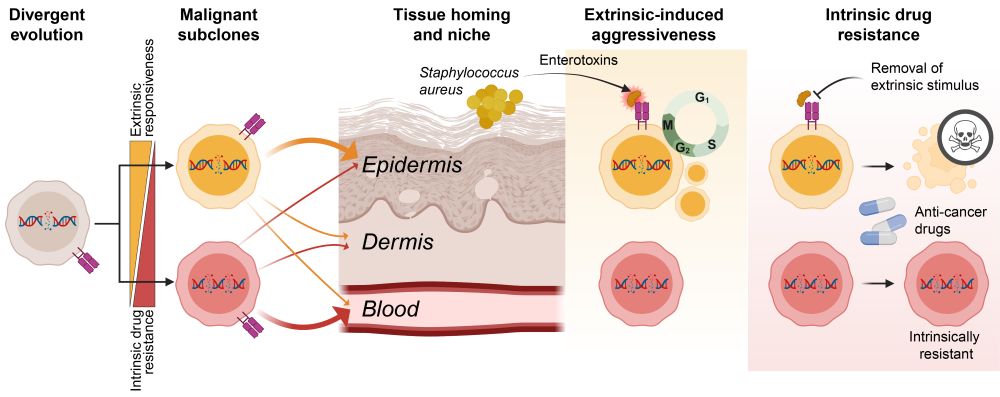
👉Leukemic CTCL patients harbor functionally distinct co-existing subclones
👉Subclones respond differently to external factors such as cytokines, bacterial infections, and cancer drugs
👉The most aggressive subclones are also most sensitive to treatment if their extrinsic stimuli are removed
2/

👉Leukemic CTCL patients harbor functionally distinct co-existing subclones
👉Subclones respond differently to external factors such as cytokines, bacterial infections, and cancer drugs
👉The most aggressive subclones are also most sensitive to treatment if their extrinsic stimuli are removed
2/
✅The answer: co-existing malignant subclones.
Let me walk you through our latest study investigating how divergent evolution drives adaptability, aggressiveness, and drug resistance of T cell cancer. 1/🧵
doi.org/10.1158/2159...

✅The answer: co-existing malignant subclones.
Let me walk you through our latest study investigating how divergent evolution drives adaptability, aggressiveness, and drug resistance of T cell cancer. 1/🧵
doi.org/10.1158/2159...

This means reducing inflammation could unmask therapeutic vulnerabilities in aggressive clones. 6/

This means reducing inflammation could unmask therapeutic vulnerabilities in aggressive clones. 6/
But it comes at a price… 5/

But it comes at a price… 5/


👉Leukemic CTCL patients harbor functionally distinct co-existing subclones
👉Subclones respond differently to external factors such as cytokines, bacterial infections, and cancer drugs
👉The most aggressive subclones are also most sensitive to treatment if their extrinsic stimuli are removed
2/

👉Leukemic CTCL patients harbor functionally distinct co-existing subclones
👉Subclones respond differently to external factors such as cytokines, bacterial infections, and cancer drugs
👉The most aggressive subclones are also most sensitive to treatment if their extrinsic stimuli are removed
2/
1. Directly through TCR-induced NFkB on malignant cells.
and
2. Indirectly through cytokines induced by SE-activation of both malignant and non-malignant T cells.

1. Directly through TCR-induced NFkB on malignant cells.
and
2. Indirectly through cytokines induced by SE-activation of both malignant and non-malignant T cells.
Highlighting the heterogeneity of SS, we found that only half the patients were dependent on JAK signaling for their resistance and half were not.

Highlighting the heterogeneity of SS, we found that only half the patients were dependent on JAK signaling for their resistance and half were not.
We show that a pool of these cytokines was also sufficient to induce drug resistance in malignant cells.

We show that a pool of these cytokines was also sufficient to induce drug resistance in malignant cells.
Moreover, only drugs that also inhibited NFkB signaling (Bortezomib & Crystal Violet) were unaffected by SE – implicating the TCR-PKC-NFkB axis.

Moreover, only drugs that also inhibited NFkB signaling (Bortezomib & Crystal Violet) were unaffected by SE – implicating the TCR-PKC-NFkB axis.

The S. aureus enterotoxins (SE) did not hamper drug uptake, efflux pumps nor interfered directly with the drugs.

The S. aureus enterotoxins (SE) did not hamper drug uptake, efflux pumps nor interfered directly with the drugs.
Recombinant SE was sufficient to induce HDACi resistance in cells but a mutated SE without superantigenic effect was not.


Recombinant SE was sufficient to induce HDACi resistance in cells but a mutated SE without superantigenic effect was not.
Importantly, treating the bacteria with SA-specific endolysin abrogated induction of resistance.

Importantly, treating the bacteria with SA-specific endolysin abrogated induction of resistance.
In this study, we ventured to figure out how.

In this study, we ventured to figure out how.

