
If, like me, you use Calibre to manage a large number of documents, then this Calibre plugin can easily help you set the mood before reading.
⬇️
If, like me, you use Calibre to manage a large number of documents, then this Calibre plugin can easily help you set the mood before reading.
⬇️

If, like me, you use Calibre to manage a large number of documents, then this Calibre plugin can easily help you set the mood before reading.
⬇️

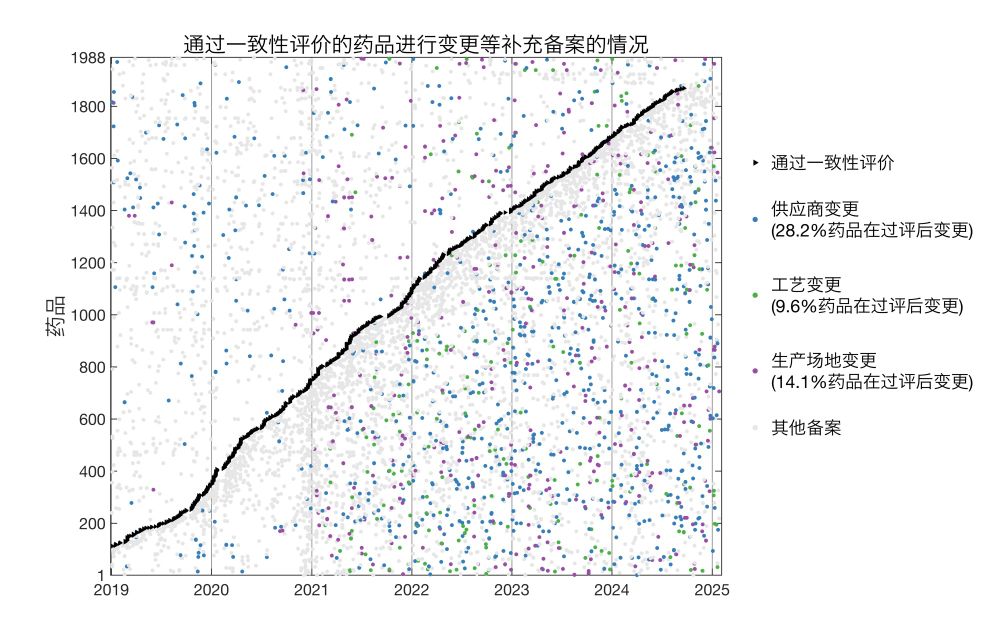
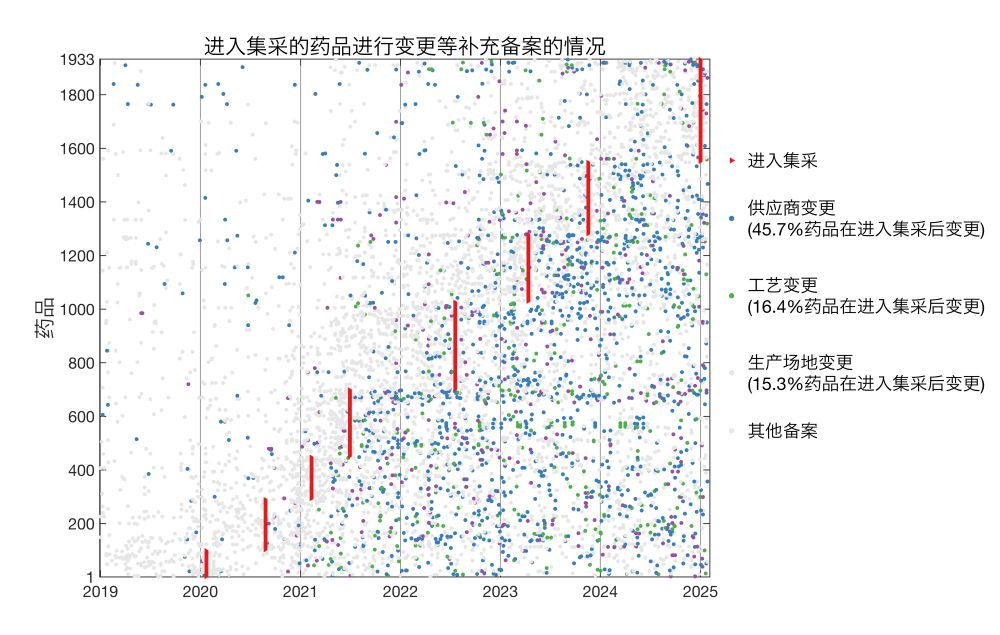
实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
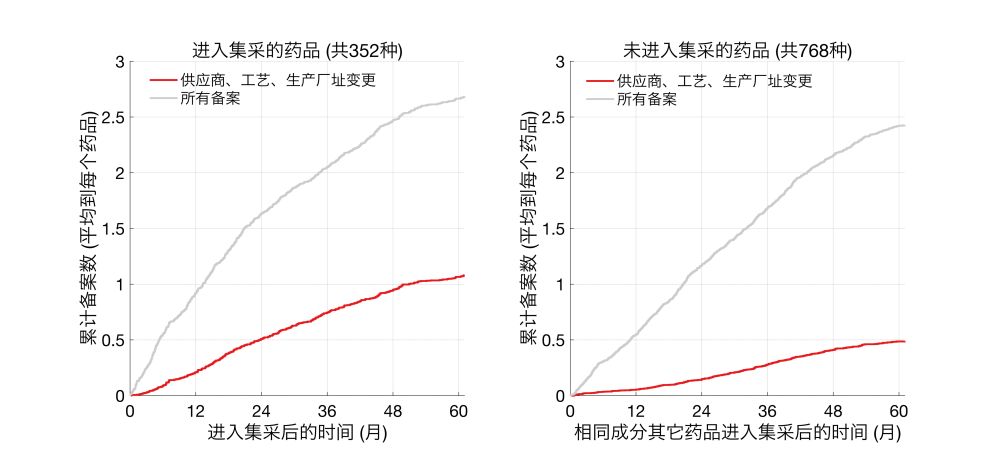
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。
实际上,一款仿制药在通过一致性评价后,可对原材料供应商、生产工艺、生产厂址等多项生产环节进行变更,而无需重新进行一致性评价,多数情况只需在省级药监部门进行备案。
我分析了国家药监局公布的2019年至今的16万余条药物补充备案,发现通过一致性评价的仿制药、进入集采的药品中,广泛存在过评后生产环节的变更。
并且,进入集采的药品,相对于同成分但未进入集采的药品,进行了更多此类变更。
这些变更并非一定会影响药效、安全性,但仍需解决如何对此进行有效监管的问题。

