https://saorisakaue.github.io/


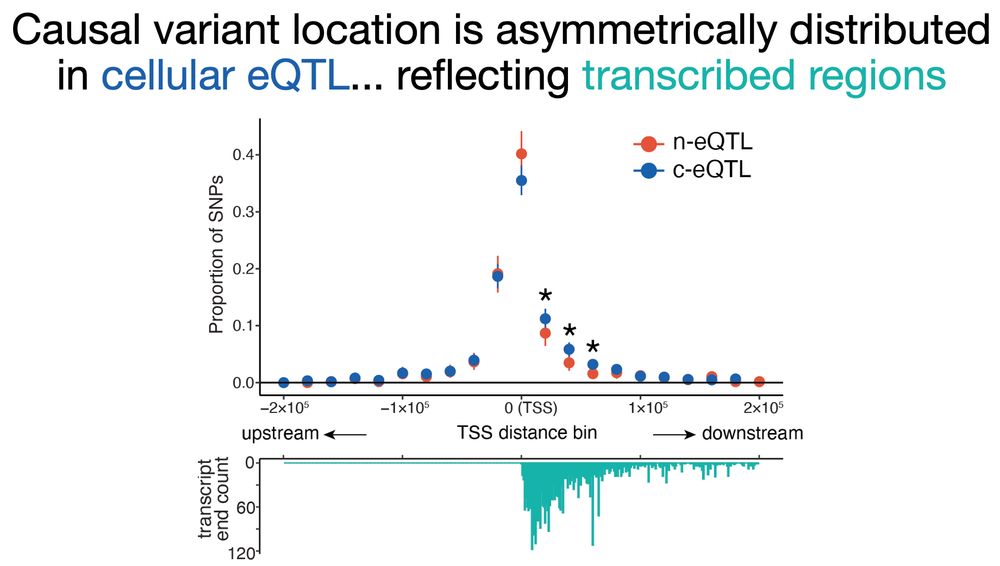
Nuclear early RNA was preferentially regulated by distal enhancers at the DNA transcription level, while cytosolic late RNA was regulated by variants within transcripts.. (7/n

Nuclear early RNA was preferentially regulated by distal enhancers at the DNA transcription level, while cytosolic late RNA was regulated by variants within transcripts.. (7/n





@soumya-boston.bsky.social on eQTL mechanisms depending on where the RNA is in the cell! @broadinstitute.org @harvardmed.bsky.social
TL;DR:Early RNA eQTL variants in the nucleus and late RNA eQTL variants in the cytosol have distinct molecular mechanism🧵

@soumya-boston.bsky.social on eQTL mechanisms depending on where the RNA is in the cell! @broadinstitute.org @harvardmed.bsky.social
TL;DR:Early RNA eQTL variants in the nucleus and late RNA eQTL variants in the cytosol have distinct molecular mechanism🧵

