
The pyrrolidine rearrangement was particularly useful for pyridazine and piperazic acid synthesis:
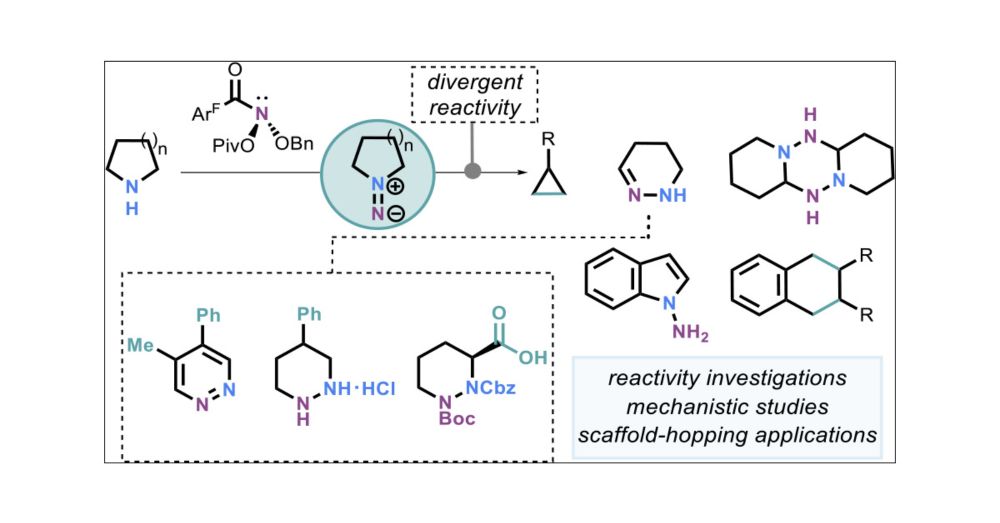
The pyrrolidine rearrangement was particularly useful for pyridazine and piperazic acid synthesis:
pubs.acs.org/doi/full/10....
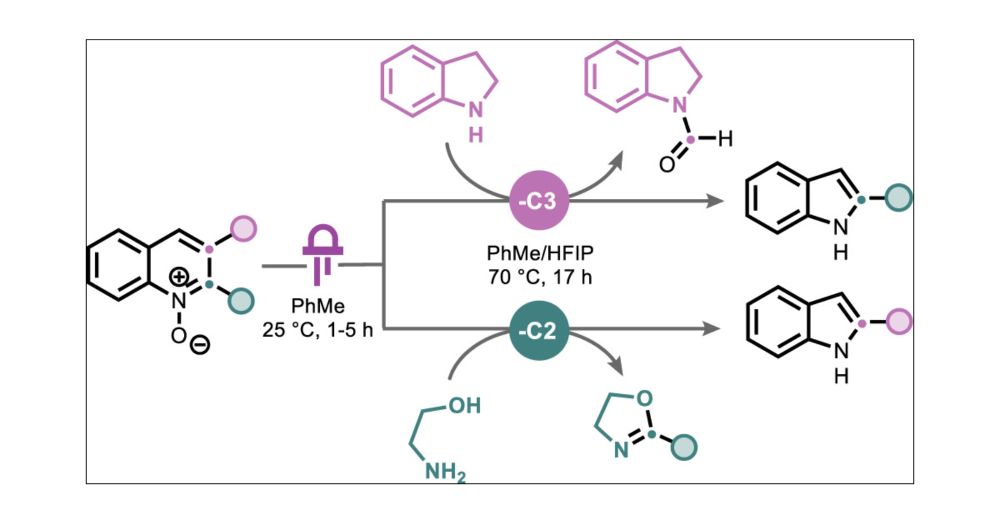
pubs.acs.org/doi/full/10....

news.uchicago.edu/story/uchica...
news.uchicago.edu/story/uchica...

