sciencedirect.com/science/arti...
Congratulations to Xin and all co-authors!
sciencedirect.com/science/arti...
Congratulations to Xin and all co-authors!
Congjun will join UC Boulder as a tenure-track Assistant Professor, and Miao will be starting at Merck as a Senior Scientist.
Wishing them both the best of luck!




Congjun will join UC Boulder as a tenure-track Assistant Professor, and Miao will be starting at Merck as a Senior Scientist.
Wishing them both the best of luck!
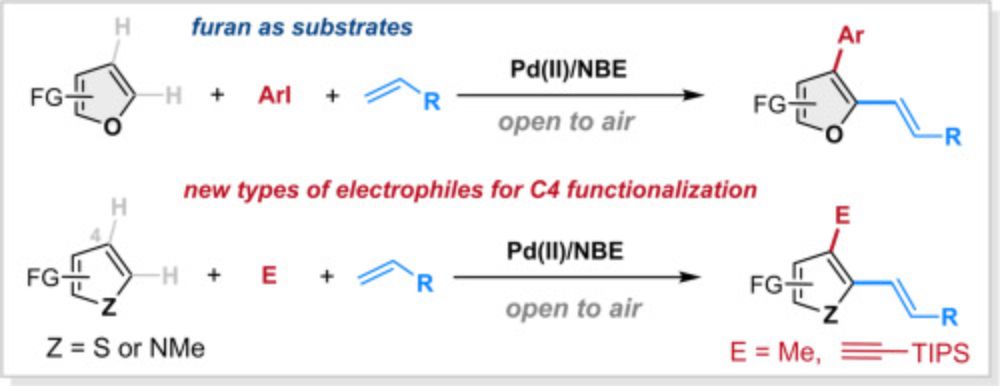
In this article, we summarized the synthesis and application of the N-methylbicyclo[2.2.1]hept-2-ene-2-carboxamide, a co-catalyst in the palladium/norbornene cooperative catalysis.
Congrats to Dr. Shinyoung Choi!
onlinelibrary.wiley.com/doi/10.1002/...

In this article, we summarized the synthesis and application of the N-methylbicyclo[2.2.1]hept-2-ene-2-carboxamide, a co-catalyst in the palladium/norbornene cooperative catalysis.
Congrats to Dr. Shinyoung Choi!
onlinelibrary.wiley.com/doi/10.1002/...
✨Lead by Kezhi, we report a branched-selective α-alkylation of aldehydes with unactivated olefins — enabled by a pyrazole mediator and a chiral Ir catalyst. Congrats!
pubs.acs.org/doi/full/10....
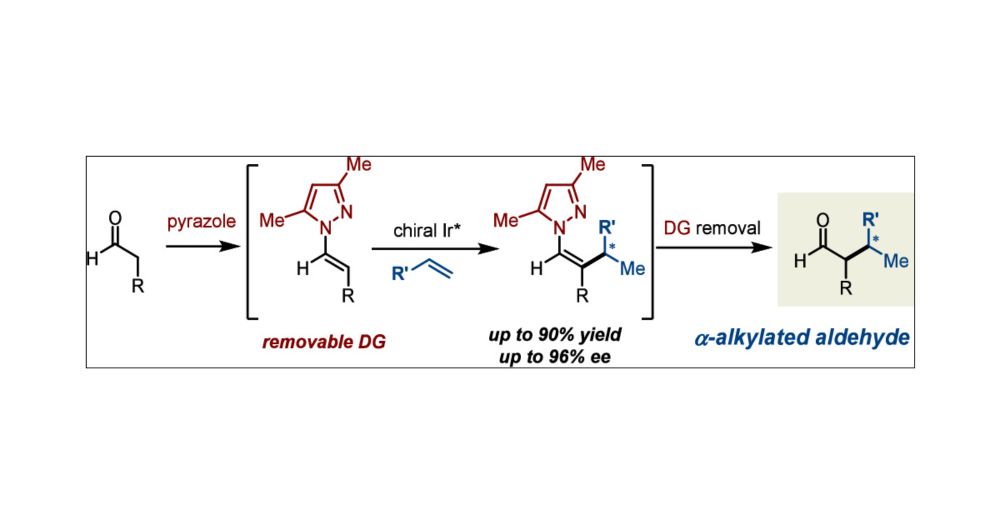
✨Lead by Kezhi, we report a branched-selective α-alkylation of aldehydes with unactivated olefins — enabled by a pyrazole mediator and a chiral Ir catalyst. Congrats!
pubs.acs.org/doi/full/10....
🔥 Our work on "Enantioconvergent carbenoid insertion into carbon−boron bonds" is now online on Nature Synthesis rdcu.be/etopg
🎉Congratulations to Qiqiang!
🙏Thanks to our collaborator Liu group
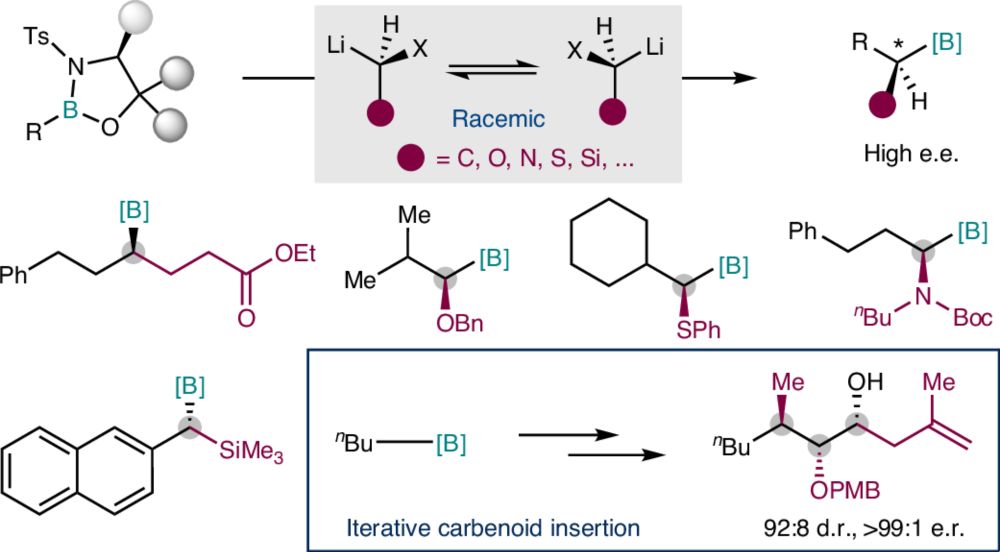
🔥 Our work on "Enantioconvergent carbenoid insertion into carbon−boron bonds" is now online on Nature Synthesis rdcu.be/etopg
🎉Congratulations to Qiqiang!
🙏Thanks to our collaborator Liu group
We have offered a general method for 1,2-difunctionlization of arenes via a differential 1,2-diborylation!
rdcu.be/esXq8
www.nature.com/articles/s41...
Congratulations to Jingfeng!
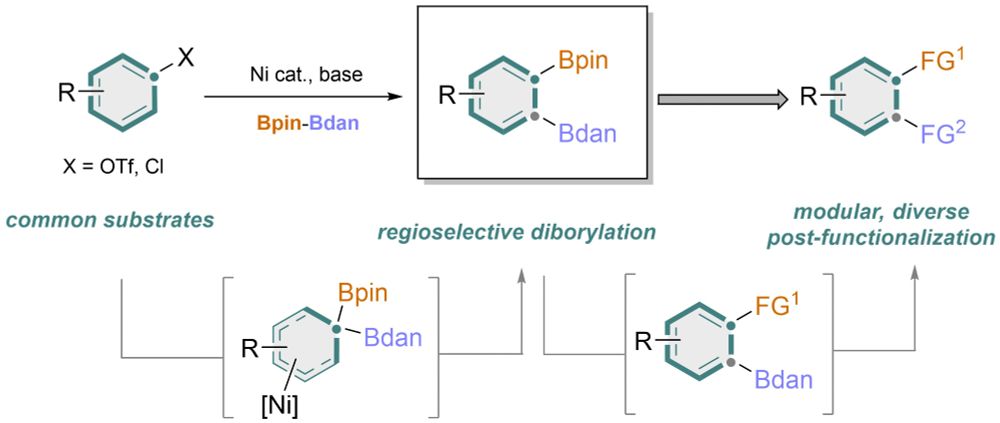
We have offered a general method for 1,2-difunctionlization of arenes via a differential 1,2-diborylation!
rdcu.be/esXq8
www.nature.com/articles/s41...
Congratulations to Jingfeng!
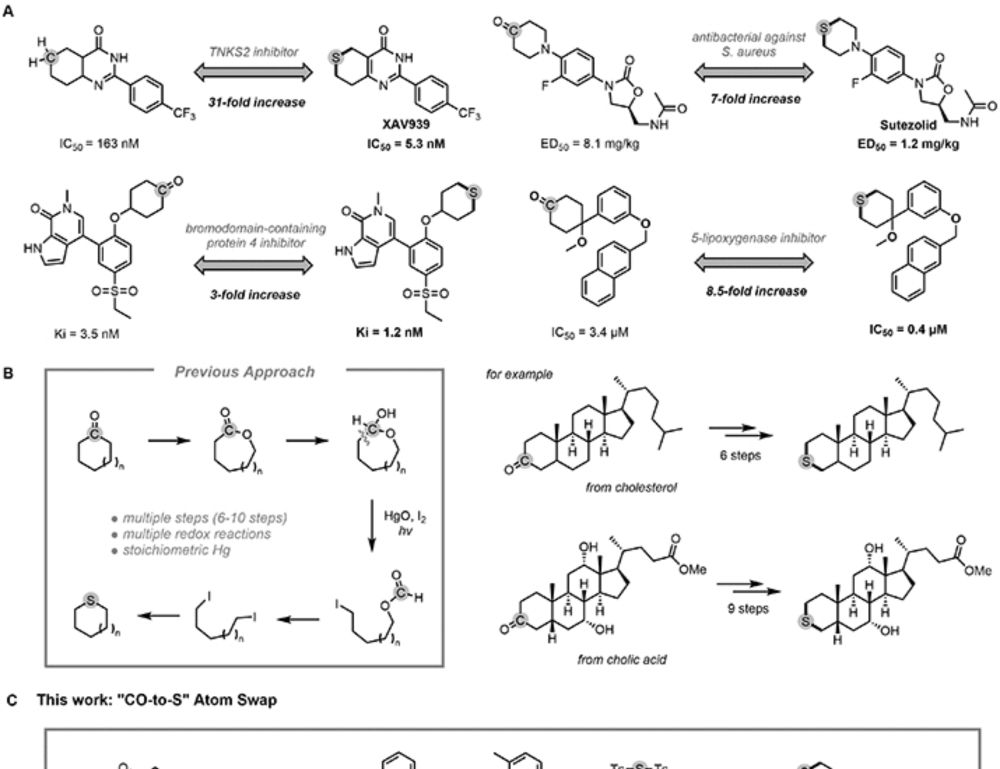
In this work, Zining from our lab developed a carbonyl-to-sulfur swap enabled by a rationally designed N′-alkyl-hydrazonamide (NAHA) reagent that promotes double C-C bond activation.
www.science.org/doi/10.1126/...
In this work, Zining from our lab developed a carbonyl-to-sulfur swap enabled by a rationally designed N′-alkyl-hydrazonamide (NAHA) reagent that promotes double C-C bond activation.
www.science.org/doi/10.1126/...
www.sciencedirect.com/science/arti...
Congratulations to Rui and Kangmin!
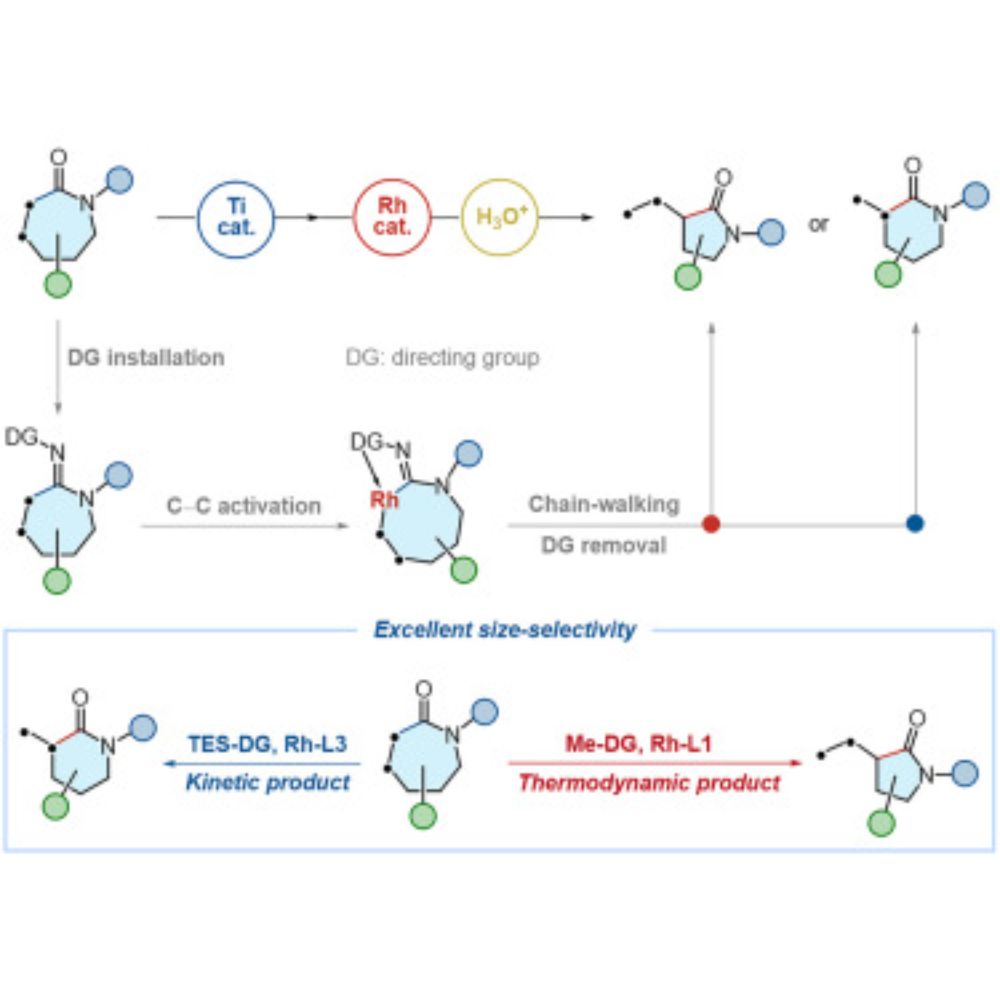
www.sciencedirect.com/science/arti...
Congratulations to Rui and Kangmin!

