
We discuss the APPOINT-PNH trial of ictacopan, a proximal complement inhibitor and the first oral therapy for PNH.
Available now on all podcast platforms.

We discuss the APPOINT-PNH trial of ictacopan, a proximal complement inhibitor and the first oral therapy for PNH.
Available now on all podcast platforms.
Part of HaemSTAR's research-focused 2 monthly webinar series hosted by the limbic
Tues 25th March 08:15 - 09:00 GMT.
thelimbic.com/uk/haematolo...

Part of HaemSTAR's research-focused 2 monthly webinar series hosted by the limbic
Tues 25th March 08:15 - 09:00 GMT.
thelimbic.com/uk/haematolo...

Pleased to release the latest episode of Don't Just Read the Abstract with Pip Nicolson. We discuss Kuhne et al.'s study in @bloodportfolio.bsky.social on the treatment of TTP without the use of plasma exchange.
There will be a follow-up episode with an author interview.

Pleased to release the latest episode of Don't Just Read the Abstract with Pip Nicolson. We discuss Kuhne et al.'s study in @bloodportfolio.bsky.social on the treatment of TTP without the use of plasma exchange.
There will be a follow-up episode with an author interview.
www.nature.com/articles/s41...

www.nature.com/articles/s41...
rpthjournal.org/article/S2475-…
I cover blinding, surrogate endpoints, clinical outcomes and thrombosis.
A brief 🧵

rpthjournal.org/article/S2475-…
I cover blinding, surrogate endpoints, clinical outcomes and thrombosis.
A brief 🧵
en.m.wikipedia.org/wiki/Ernest_...

en.m.wikipedia.org/wiki/Ernest_...





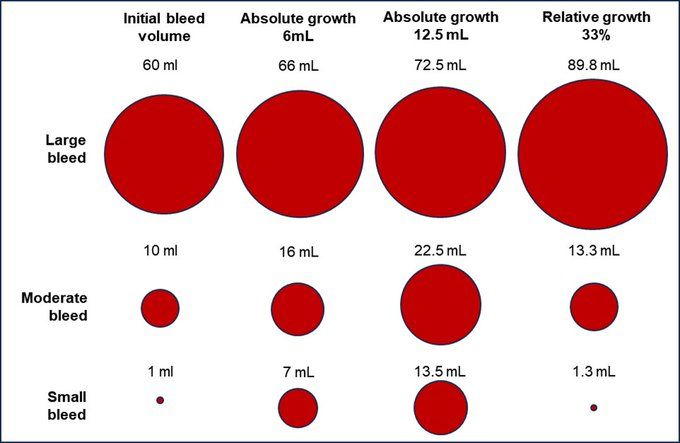
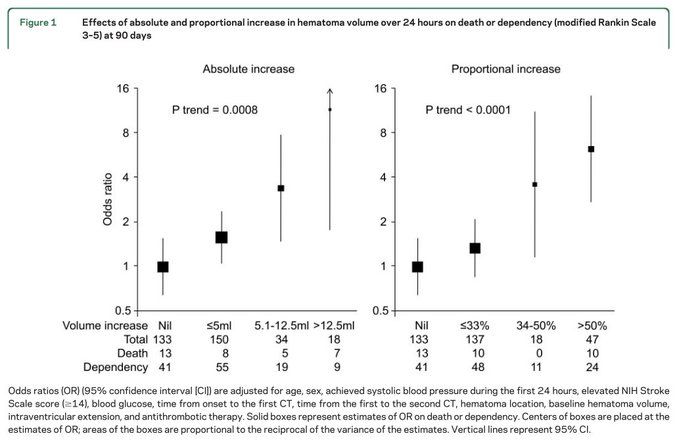
Many point out that 30 days is too short to look for survival effects of acute interventions but this is all we have to go on.
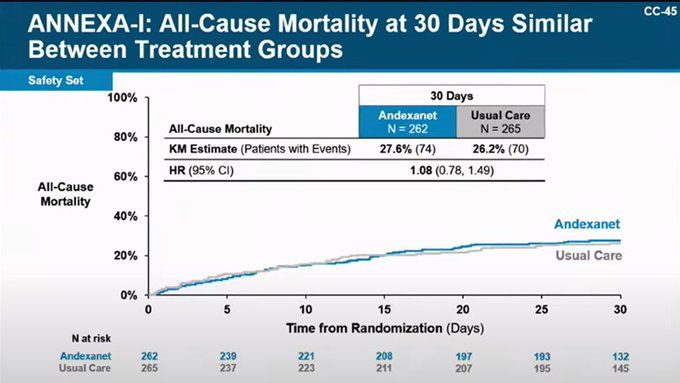
Many point out that 30 days is too short to look for survival effects of acute interventions but this is all we have to go on.
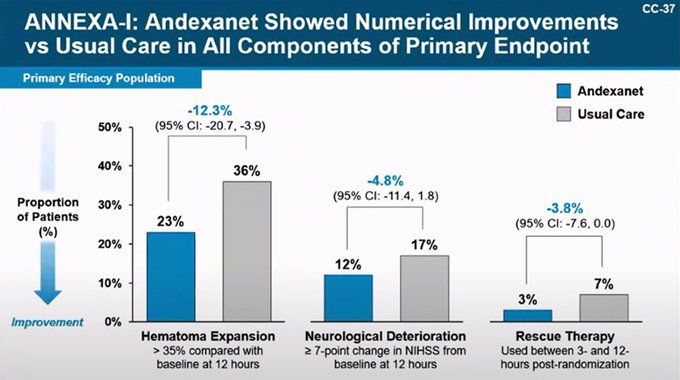








Final figures (based on n=471 FDA safety set): 14.6% vs 6.9%, stroke 5% vs 0.4%.

Final figures (based on n=471 FDA safety set): 14.6% vs 6.9%, stroke 5% vs 0.4%.

