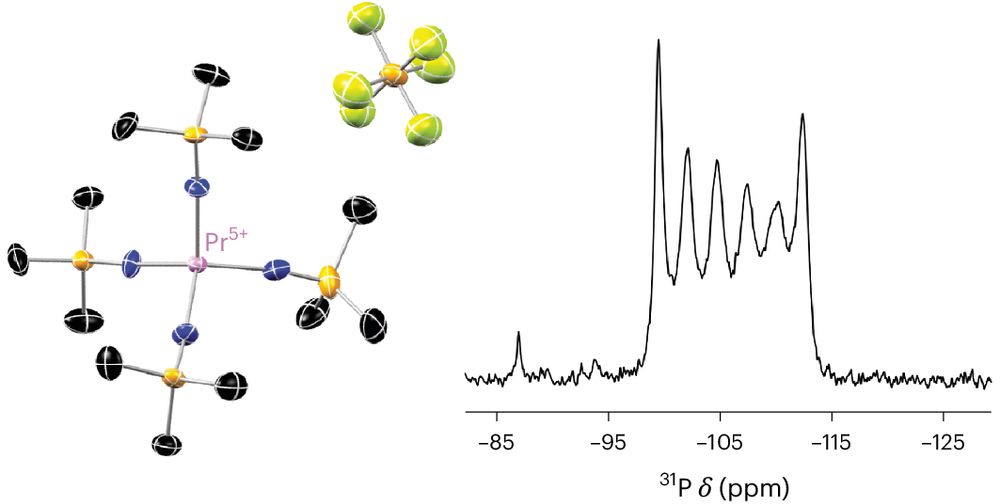
Interested in how fine tuning of reaction conditions and judicious choice of oxidising agent can lead to isolation of rare Pr(IV) complexes...the answer is here! Thanks to all my collaborators for this wonderful chemistry. @ChemicalScience.rsc.org
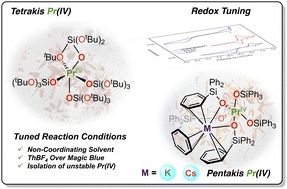
Interested in how fine tuning of reaction conditions and judicious choice of oxidising agent can lead to isolation of rare Pr(IV) complexes...the answer is here! Thanks to all my collaborators for this wonderful chemistry. @ChemicalScience.rsc.org
Interested in single crystal to single crystal ring opening reaction and participation of 4f orbital here is recent article @natchem.nature.com by Schelter’s group and team!!
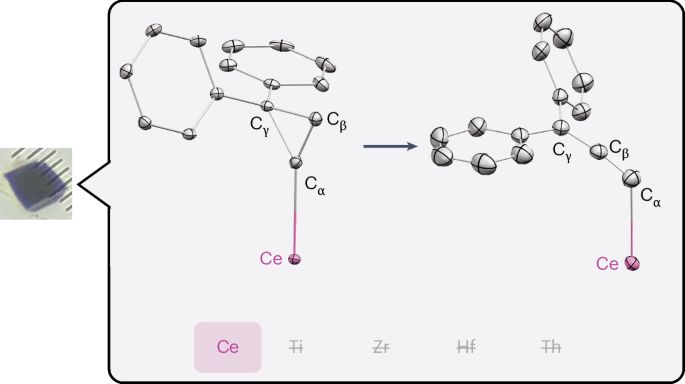
Interested in single crystal to single crystal ring opening reaction and participation of 4f orbital here is recent article @natchem.nature.com by Schelter’s group and team!!
www.nature.com/articles/s41...
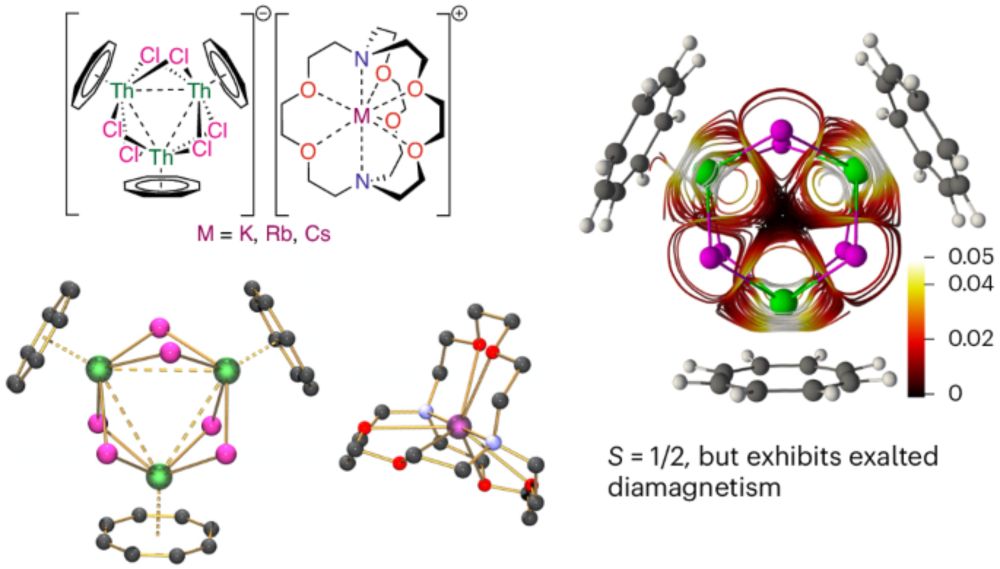
www.nature.com/articles/s41...