Michael Browning
@mikebrowning.bsky.social
Researcher and psychiatrist in Oxford
Hello, in case you are interested, the study is complete and the paper is published here: www.thelancet.com/journals/lan...

Pramipexole augmentation for the acute phase of treatment-resistant, unipolar depression: a placebo-controlled, double-blind, randomised trial in the UK
In this trial involving participants with treatment-resistant depression, pramipexole
augmentation of antidepressant treatment, at a target dose of 2·5 mg, demonstrated
a reduction in symptoms relativ...
www.thelancet.com
October 20, 2025 at 4:26 PM
Hello, in case you are interested, the study is complete and the paper is published here: www.thelancet.com/journals/lan...
It is generally higher than you see with ssris. This paper reports the odds of dropping out due to intolerance for lots of antidepressants vs placebo (page 154 of appendix). By comparison, the or in our study was 4.4.
www.thelancet.com/article/S014...
www.thelancet.com/article/S014...
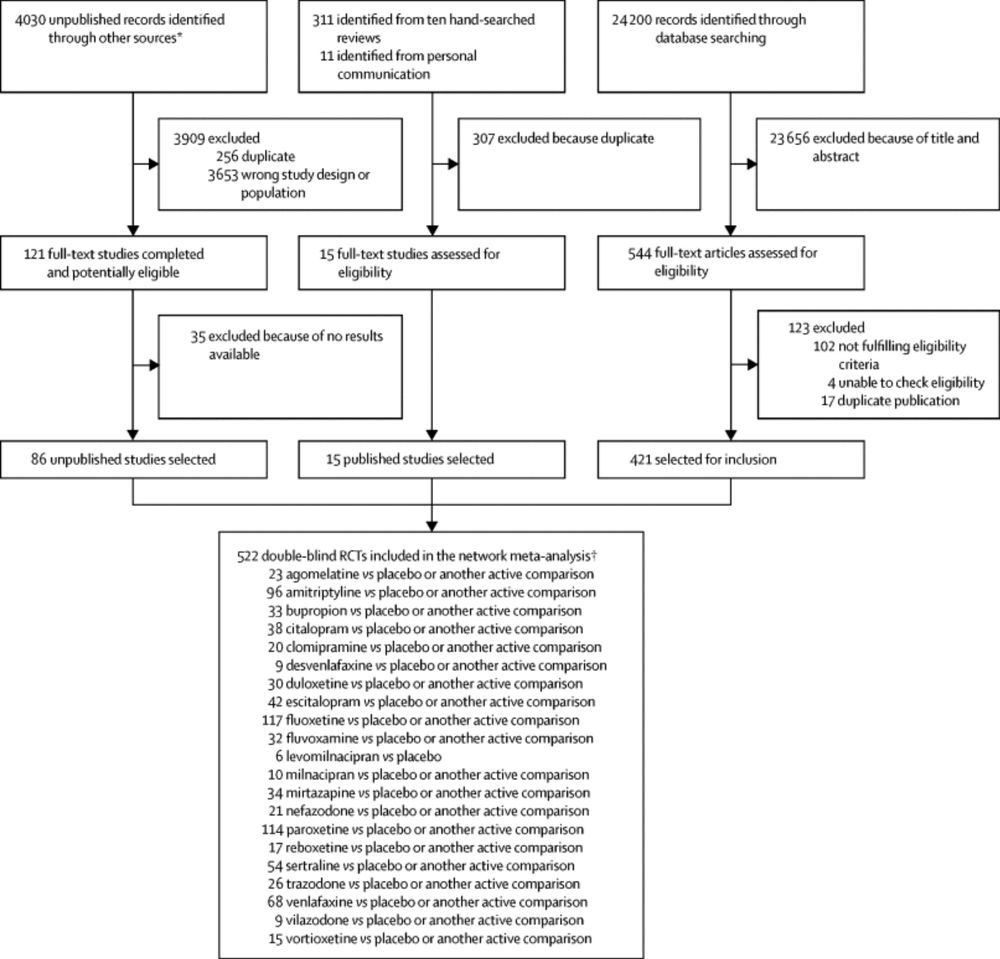
Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis
All antidepressants were more efficacious than placebo in adults with major depressive
disorder. Smaller differences between active drugs were found when placebo-controlled
trials were included in the...
www.thelancet.com
June 30, 2025 at 1:47 PM
It is generally higher than you see with ssris. This paper reports the odds of dropping out due to intolerance for lots of antidepressants vs placebo (page 154 of appendix). By comparison, the or in our study was 4.4.
www.thelancet.com/article/S014...
www.thelancet.com/article/S014...
GRATEFUL to @nihr.bsky.social for funding this work and @oxhealthbrc.bsky.social for support.
Pramipexole is clearly not a first line treatment for depression, but these results demonstrate that it can be a useful option for our most treatment-resistant patients! 6/6
Pramipexole is clearly not a first line treatment for depression, but these results demonstrate that it can be a useful option for our most treatment-resistant patients! 6/6
June 30, 2025 at 3:54 AM
GRATEFUL to @nihr.bsky.social for funding this work and @oxhealthbrc.bsky.social for support.
Pramipexole is clearly not a first line treatment for depression, but these results demonstrate that it can be a useful option for our most treatment-resistant patients! 6/6
Pramipexole is clearly not a first line treatment for depression, but these results demonstrate that it can be a useful option for our most treatment-resistant patients! 6/6
TRANSPARENCY: There are challenges to using pramipexole: 20% stopped pramipexole due to side effects vs 5% placebo. Most common: nausea, headache, dizziness, sleep problems. 5/6
June 30, 2025 at 3:54 AM
TRANSPARENCY: There are challenges to using pramipexole: 20% stopped pramipexole due to side effects vs 5% placebo. Most common: nausea, headache, dizziness, sleep problems. 5/6
HYPOTHESIS: We targeted dopamine D2/3 receptors with pramipexole instead of the usual serotonergic approach. Dopamine is crucial for reward/pleasure - and anhedonia (can't feel pleasure) is often what persists even after other depression symptoms improve. 4/6
June 30, 2025 at 3:54 AM
HYPOTHESIS: We targeted dopamine D2/3 receptors with pramipexole instead of the usual serotonergic approach. Dopamine is crucial for reward/pleasure - and anhedonia (can't feel pleasure) is often what persists even after other depression symptoms improve. 4/6
Background: About 30% of our patients don't respond to first-line antidepressants. We recruited people with chronic depression (median 8.5 years) who had tried 3-4 medications without success. These are the patients we struggle to help most. 3/6
June 30, 2025 at 3:54 AM
Background: About 30% of our patients don't respond to first-line antidepressants. We recruited people with chronic depression (median 8.5 years) who had tried 3-4 medications without success. These are the patients we struggle to help most. 3/6
FINDINGS: In our randomized controlled trial (n=150), patients receiving pramipexole showed:
• 6.4-point reduction in QIDS scores vs 2.4 for placebo (p<0.0001)
• 44% response rate vs 16% placebo
• 28% remission vs 8% placebo Effect size d=0.87 - this is substantial! 2/6
• 6.4-point reduction in QIDS scores vs 2.4 for placebo (p<0.0001)
• 44% response rate vs 16% placebo
• 28% remission vs 8% placebo Effect size d=0.87 - this is substantial! 2/6
June 30, 2025 at 3:54 AM
FINDINGS: In our randomized controlled trial (n=150), patients receiving pramipexole showed:
• 6.4-point reduction in QIDS scores vs 2.4 for placebo (p<0.0001)
• 44% response rate vs 16% placebo
• 28% remission vs 8% placebo Effect size d=0.87 - this is substantial! 2/6
• 6.4-point reduction in QIDS scores vs 2.4 for placebo (p<0.0001)
• 44% response rate vs 16% placebo
• 28% remission vs 8% placebo Effect size d=0.87 - this is substantial! 2/6
It's love Rick.
March 13, 2025 at 6:41 PM
It's love Rick.

