
Website: https://luo.chem.utah.edu/
advanced.onlinelibrary.wiley.com/doi/10.1002/...
@utahchemistry.bsky.social @NSF_CSOE

advanced.onlinelibrary.wiley.com/doi/10.1002/...
@utahchemistry.bsky.social @NSF_CSOE





, and postdoc Mike Pence, are bringing automation to the quantitative analysis class @utahchemistry.bsky.social ! Thanks @rescorp.org @NSF for funding support! #modernize analytical chemistry labs!


, and postdoc Mike Pence, are bringing automation to the quantitative analysis class @utahchemistry.bsky.social ! Thanks @rescorp.org @NSF for funding support! #modernize analytical chemistry labs!
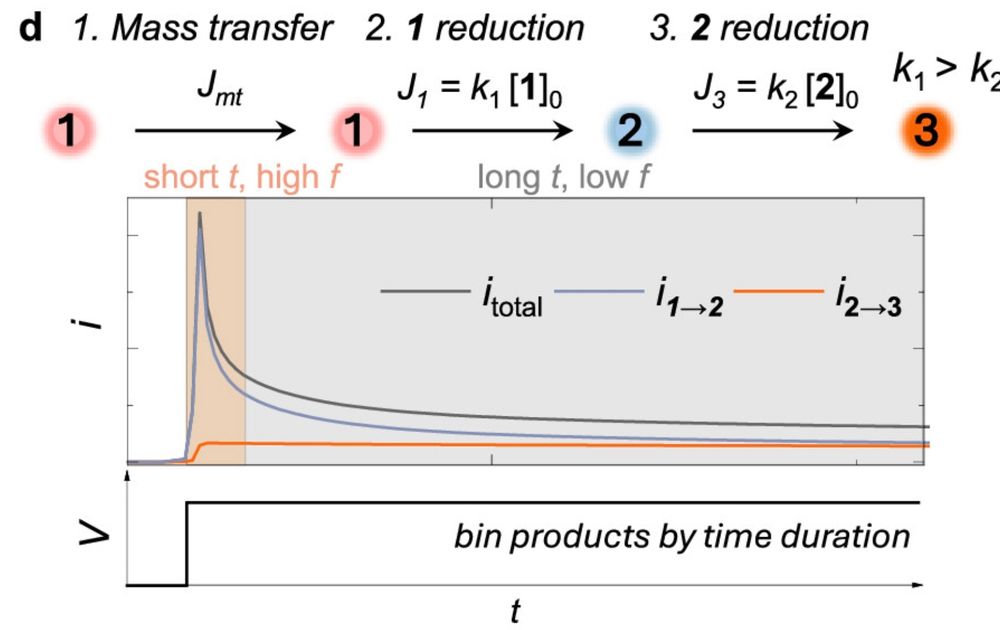
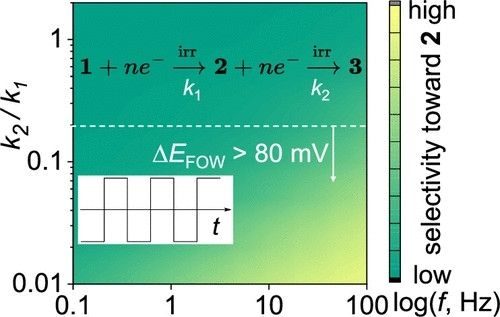




@minteerlab.bsky.social , TD, Siegi, and Lane @bakergrp.bsky.social for the continued support!

@minteerlab.bsky.social , TD, Siegi, and Lane @bakergrp.bsky.social for the continued support!

pubs.rsc.org/en/content/a...
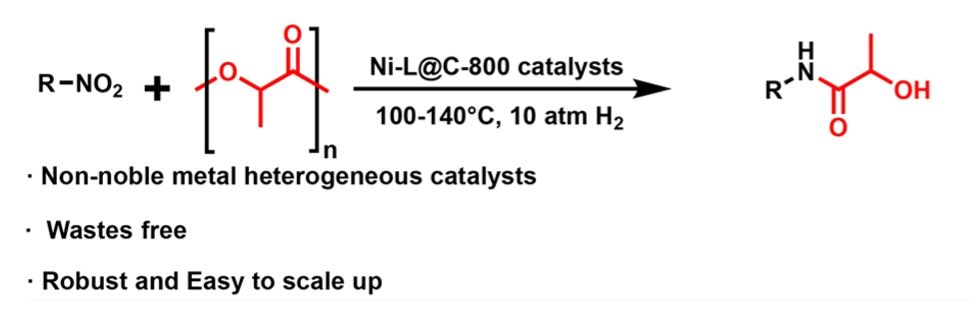
pubs.rsc.org/en/content/a...

and computational studies with Zhenfei Liu @waynestatechem
confirmed the surface binding geometry and the surface pathway.
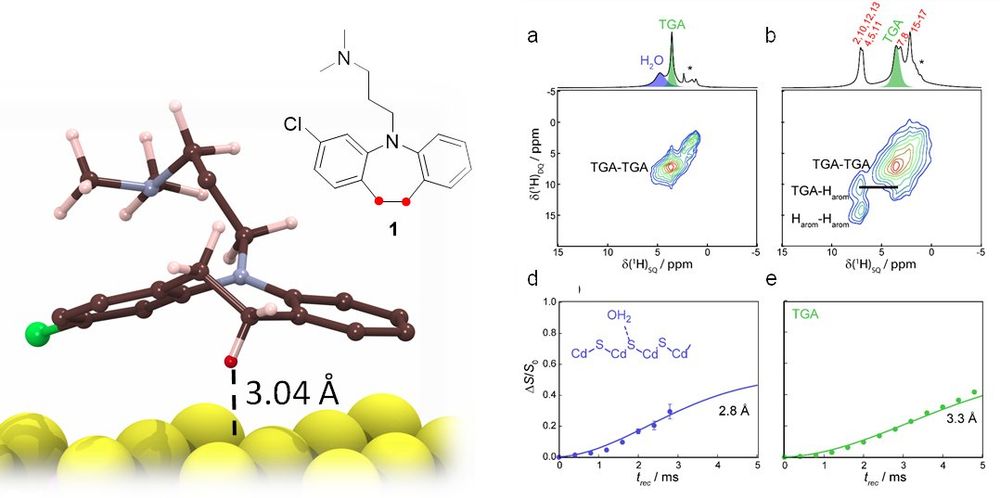
and computational studies with Zhenfei Liu @waynestatechem
confirmed the surface binding geometry and the surface pathway.

