
@michelrickhaus.bsky.social @avj-chem.bsky.social @rickhauslab.bsky.social

@michelrickhaus.bsky.social @avj-chem.bsky.social @rickhauslab.bsky.social
In our latest @jacs.acspublications.org at 🇨🇭UNIGE, we ferret out vastly different organisations in wet vs dry solvents that lead to identical macrostructures
👉 doi.org/10.1021/jacs.4c17024 #pisky #chemsky
@rickhauslab.bsky.social
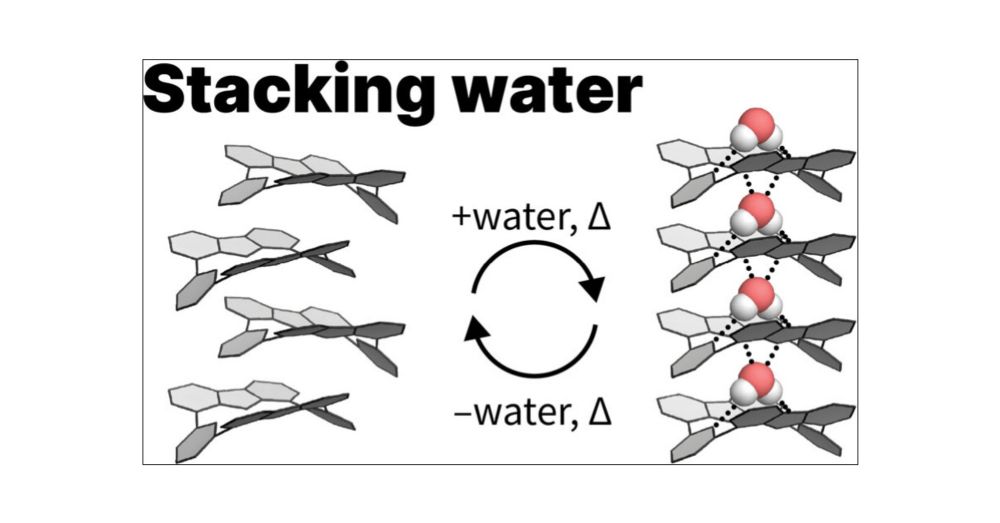
In our latest @jacs.acspublications.org at 🇨🇭UNIGE, we ferret out vastly different organisations in wet vs dry solvents that lead to identical macrostructures
👉 doi.org/10.1021/jacs.4c17024 #pisky #chemsky
@rickhauslab.bsky.social
@uzhchemistry.bsky.social

@uzhchemistry.bsky.social
We're especially proud of this one: Supramolecular chemistry lends a helping hand in developing new photo-generated molecular qubits. A fantastic collaboration with the @sabine-richert.bsky.social group!
www.nature.com/articles/s41...

We're especially proud of this one: Supramolecular chemistry lends a helping hand in developing new photo-generated molecular qubits. A fantastic collaboration with the @sabine-richert.bsky.social group!
www.nature.com/articles/s41...
Authors: Joseph F. Woods, Kai Zhang, Joëlle Peterschmitt, Olivier Blacque, Céline Besnard, Gustavo Santiso-Quinones, Laura Samperisi, Andreas Vargas Jentzsch, Michel Rickhaus
DOI: 10.26434/chemrxiv-2024-twhrl
Authors: Joseph F. Woods, Kai Zhang, Joëlle Peterschmitt, Olivier Blacque, Céline Besnard, Gustavo Santiso-Quinones, Laura Samperisi, Andreas Vargas Jentzsch, Michel Rickhaus
DOI: 10.26434/chemrxiv-2024-twhrl

