lcs.epfl.ch

#chemsky #EPFL

#chemsky #EPFL
#chemsky #EPFL

#chemsky #EPFL

doi.org/10.1021/acs....
What next? With all these nice tools developed, we are eager to collaborate with chemical biologists for applications!

doi.org/10.1021/acs....
What next? With all these nice tools developed, we are eager to collaborate with chemical biologists for applications!
We are hiring! New opening for a W2 Professor in "experimental inorganic chemistry" @unibonn.bsky.social
Deadline Oct. 10 t.co/EqMLA0fCuC

We are hiring! New opening for a W2 Professor in "experimental inorganic chemistry" @unibonn.bsky.social
Deadline Oct. 10 t.co/EqMLA0fCuC
#chemsky #EPFL

#chemsky #EPFL
Please repost...
www.epfl.ch/about/workin...

Please repost...
www.epfl.ch/about/workin...
jobs.unige.ch/www/wd_porta...
jobs.unige.ch/www/wd_porta...
doi.org/10.1002/anie...
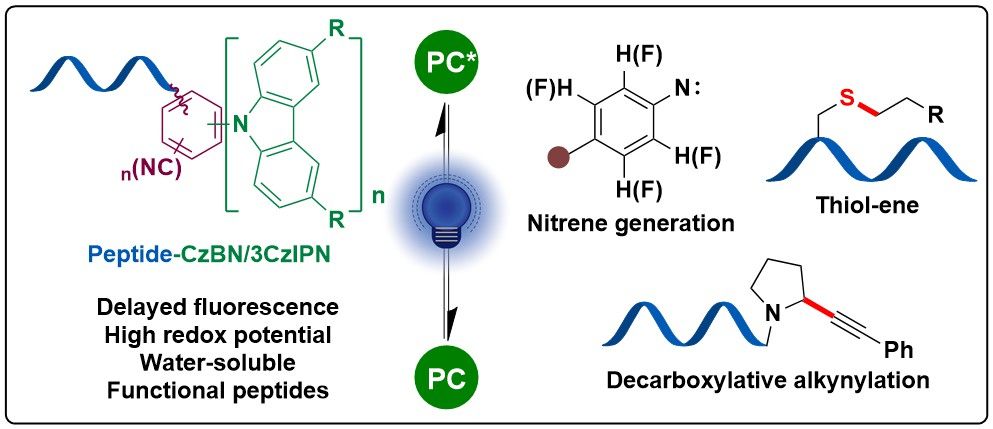
doi.org/10.1002/anie...
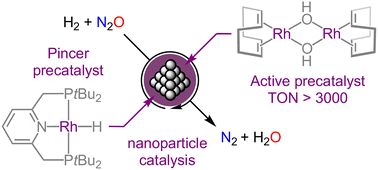
@irenebenzop.bsky.social #chemsky #EPFL

@irenebenzop.bsky.social #chemsky #EPFL
#chemsky #EPFL

#chemsky #EPFL
This work of Najung, Jonas and Elija has just been accepted in @angewandtechemie.bsky.social !
doi.org/10.1002/anie...

This work of Najung, Jonas and Elija has just been accepted in @angewandtechemie.bsky.social !
doi.org/10.1002/anie...
Meet the 2025 Class: cen.acs.org/people/profi...
#CENT12 #chemsky🧪
pubs.acs.org/doi/10.1021/...
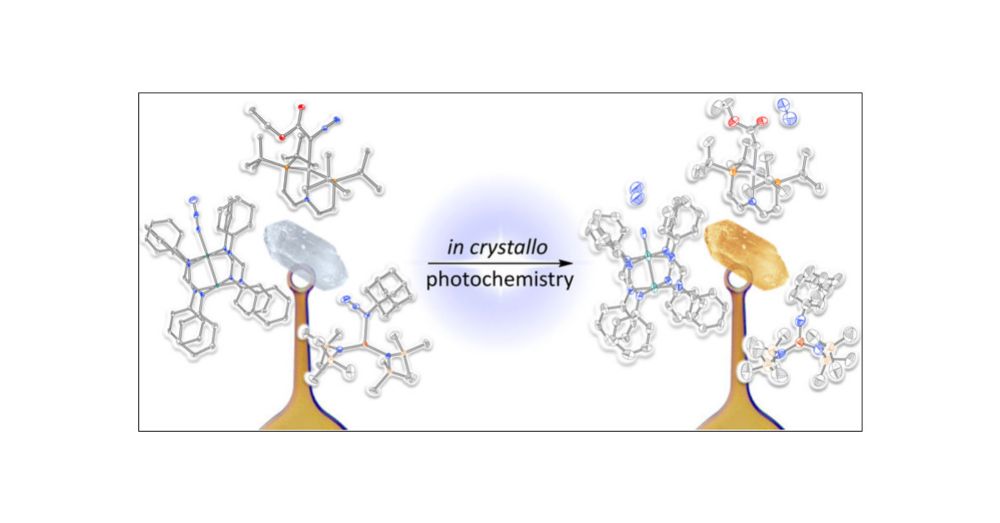
pubs.acs.org/doi/10.1021/...

my.corehr.com/pls/uoxrecru...
my.corehr.com/pls/uoxrecru...
www.epfl.ch/labs/lcso/gr...

www.epfl.ch/labs/lcso/gr...
#chemsky #EPFL 🧪

#chemsky #EPFL 🧪
@unibe.ch #openaccess #TeamWorkMakestheDreamWork
www.nature.com/articles/s44...


@unibe.ch #openaccess #TeamWorkMakestheDreamWork
www.nature.com/articles/s44...
#chemsky #EPFL 🧪
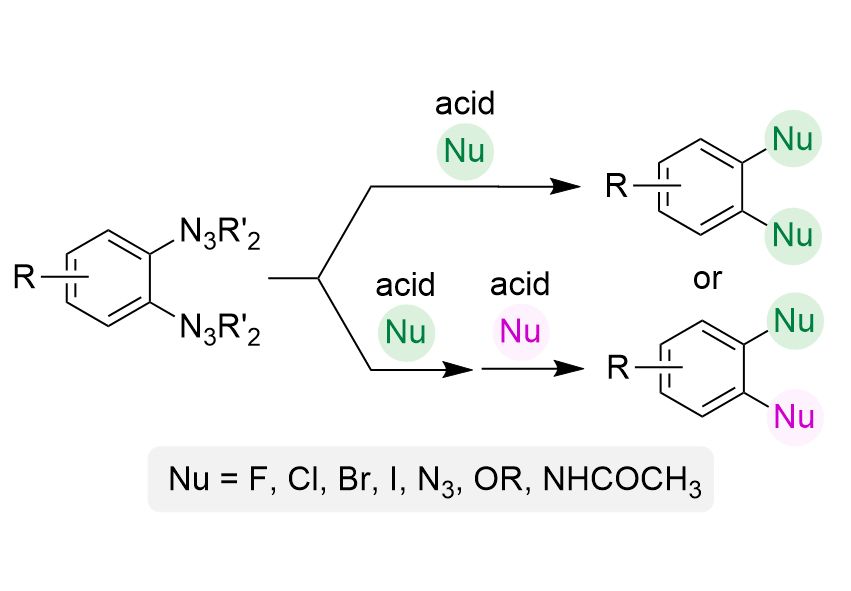
#chemsky #EPFL 🧪
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
#chemsky #EPFL 🧪

#chemsky #EPFL 🧪
Serving as Editor-in-Chief at Science was fascinating. I greatly enjoyed working with talented and committed editorial, news, graphics, and production staff. But the inside look into scientific publishing and AAAS was also deeply disillusioning.


