pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
Check out our latest paper on “Precise Carboxylic Acid-Functionalized Polyesters in Reprocessable Vitrimers” now out in JACS! ♻️👇
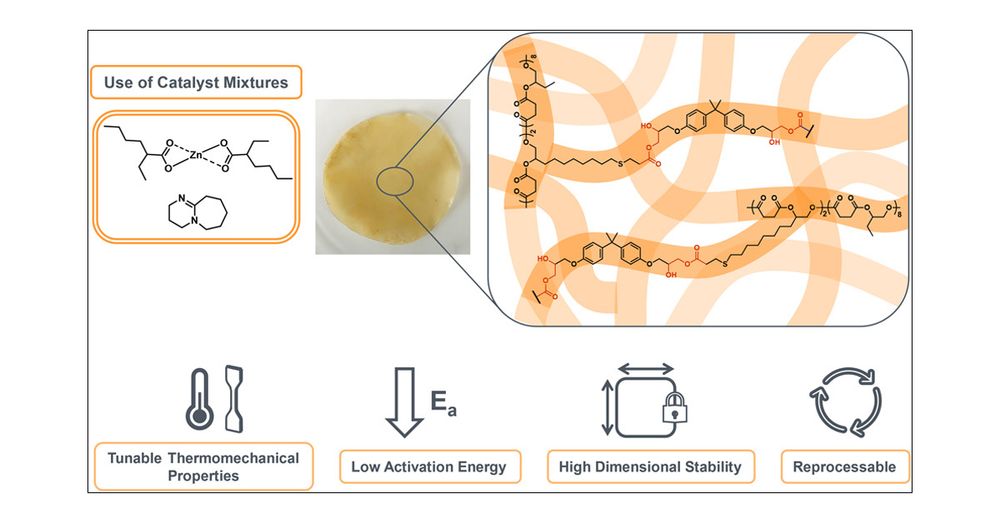
Check out our latest paper on “Precise Carboxylic Acid-Functionalized Polyesters in Reprocessable Vitrimers” now out in JACS! ♻️👇
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...
A new study analysed the odours of ancient Egyptian mummies, detecting woody, spicy, and sweet scents.
Using chemistry & sensory panels, researchers linked smells to embalming materials & even pesticides.
🔗 doi.org/10.1021/jacs...
#SciComm #Archaeology

A new study analysed the odours of ancient Egyptian mummies, detecting woody, spicy, and sweet scents.
Using chemistry & sensory panels, researchers linked smells to embalming materials & even pesticides.
🔗 doi.org/10.1021/jacs...
#SciComm #Archaeology
JACS on how to teach a low-coordinate MgO fragment to reversibly activate dihydrogen under very mild conditions: pubs.acs.org/doi/10.1021/...
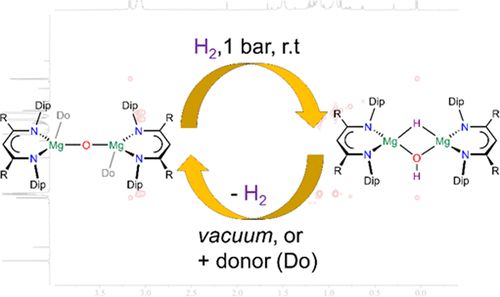
JACS on how to teach a low-coordinate MgO fragment to reversibly activate dihydrogen under very mild conditions: pubs.acs.org/doi/10.1021/...

#openaccess #chemsky
#openaccess #chemsky
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
@gemmagransbury.bsky.social

@gemmagransbury.bsky.social




