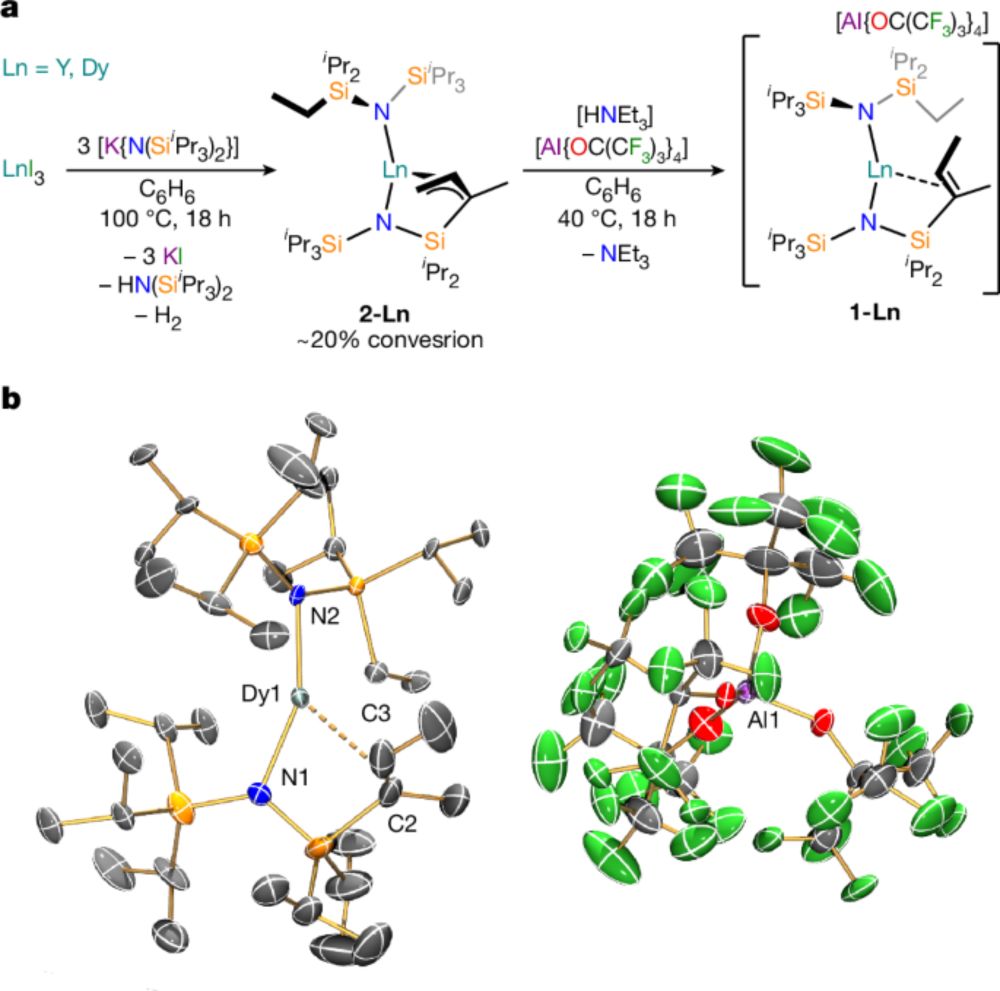
www.nature.com/articles/s41...

Special congratulations to Rebecca, Kevin and Nick for their synthetic work.
rdcu.be/eBcJt

Special congratulations to Rebecca, Kevin and Nick for their synthetic work.
rdcu.be/eBcJt





chemrxiv.org/engage/chemr...

chemrxiv.org/engage/chemr...
www.nature.com/articles/s41...
www.nature.com/articles/s41...
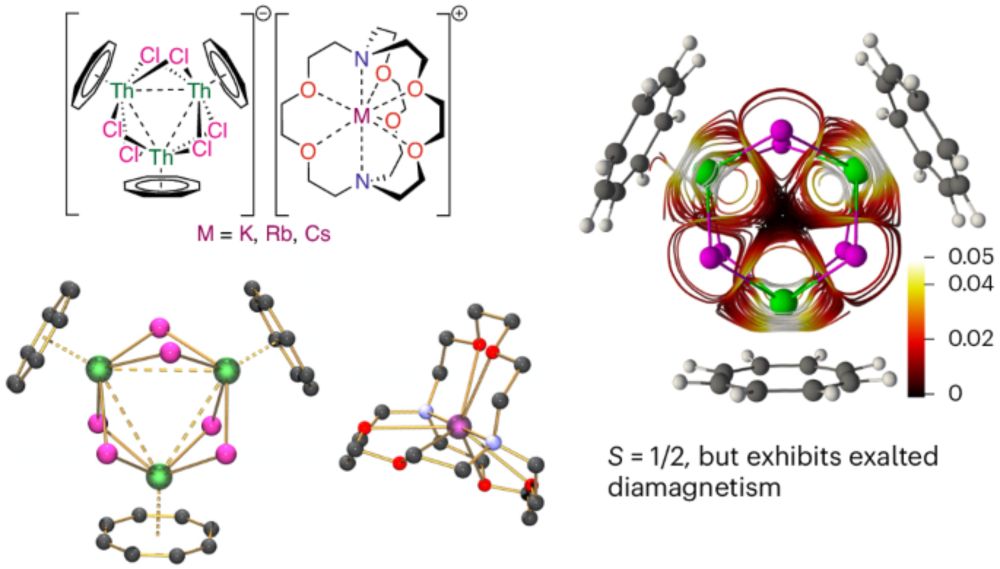
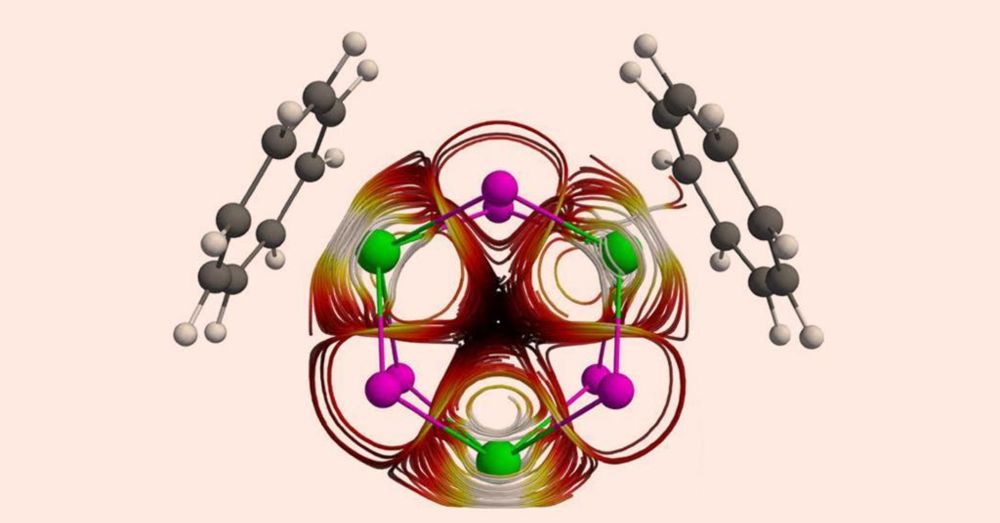
@angewandtechemie.bsky.social @maxdietz.bsky.social @amelia-swarbrook.bsky.social
doi.org/10.1002/anie...

@angewandtechemie.bsky.social @maxdietz.bsky.social @amelia-swarbrook.bsky.social
doi.org/10.1002/anie...
#ChemSky
#ChemSky
www.nature.com/articles/s41...

www.nature.com/articles/s41...
chemsky🧪
www.science.org/doi/10.1126/...

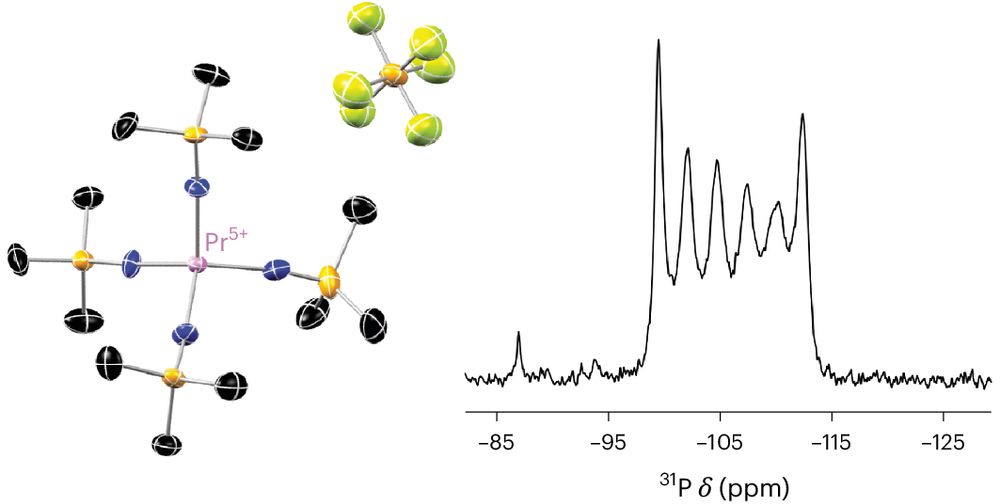
Challenging synthesis and some interesting new main group architectures making an appearance for the first time on an f-element.
#Chemistry #Actinides #f-elements
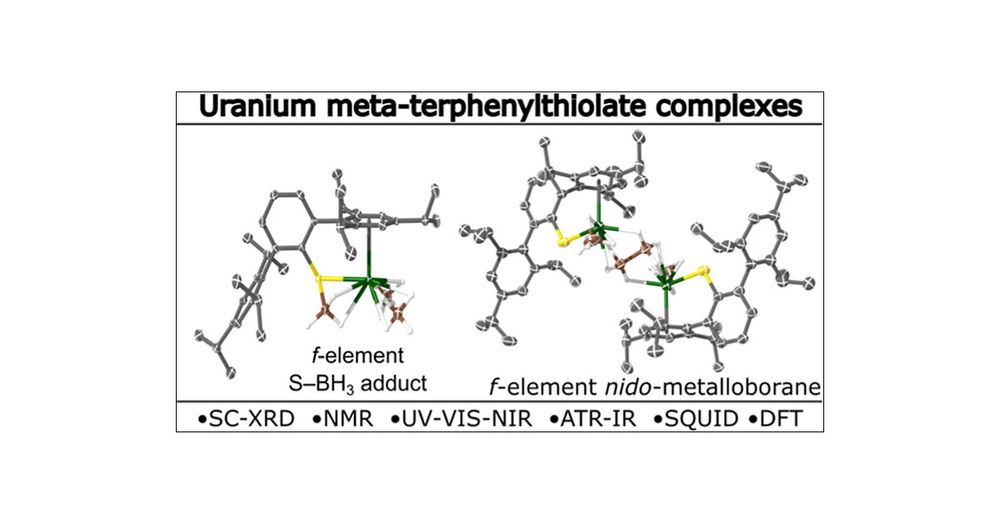
Challenging synthesis and some interesting new main group architectures making an appearance for the first time on an f-element.
#Chemistry #Actinides #f-elements