
Jennifer Juno
@jenjuno.viralvaxlab.com
🇨🇦 in 🇦🇺 Scientist. Geek. Feminist. She/her. Lab head (viralvaxlab.com) at the Doherty Institute and University of Melbourne, interested in all things CD4/TFH/vaccine and virus-related. I stare at dots a lot.
What do you need them for, Julie? We might have other lines that would work for your experiments.
March 20, 2025 at 6:36 AM
What do you need them for, Julie? We might have other lines that would work for your experiments.
Unfortunately, Melbourne isn't going to be quite as seamless of an experience...
February 28, 2025 at 4:46 AM
Unfortunately, Melbourne isn't going to be quite as seamless of an experience...
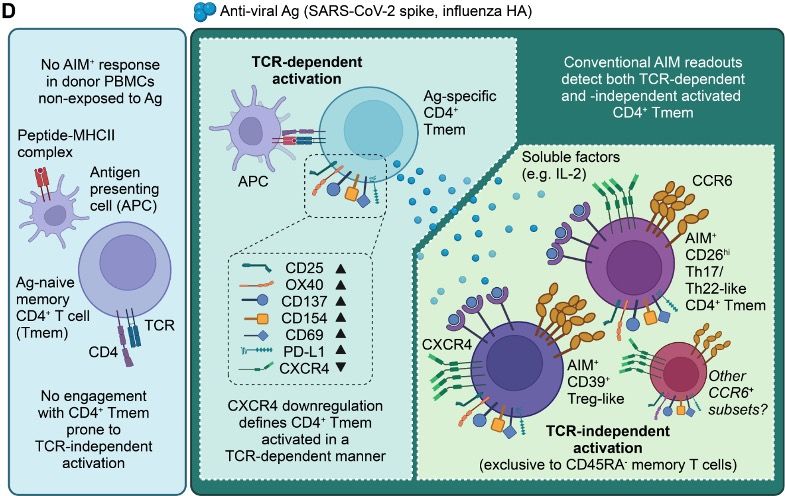
9/9 Thanks to all our collaborators who helped out with the study, and congrats to Mitchell, who persevered through a lot of possible activation marker combinations!
December 20, 2024 at 3:39 AM
9/9 Thanks to all our collaborators who helped out with the study, and congrats to Mitchell, who persevered through a lot of possible activation marker combinations!
8/9 In short, we suggest in vitro stimulation activates ag-specific T cells, which secrete cytokines and activate Treg and Th17/22 cells. Without careful phenotyping, all of these cells can get picked up as AIM+, accounting for the Th17-like memory cells found in many virus-specific AIM datasets.
December 20, 2024 at 3:39 AM
8/9 In short, we suggest in vitro stimulation activates ag-specific T cells, which secrete cytokines and activate Treg and Th17/22 cells. Without careful phenotyping, all of these cells can get picked up as AIM+, accounting for the Th17-like memory cells found in many virus-specific AIM datasets.
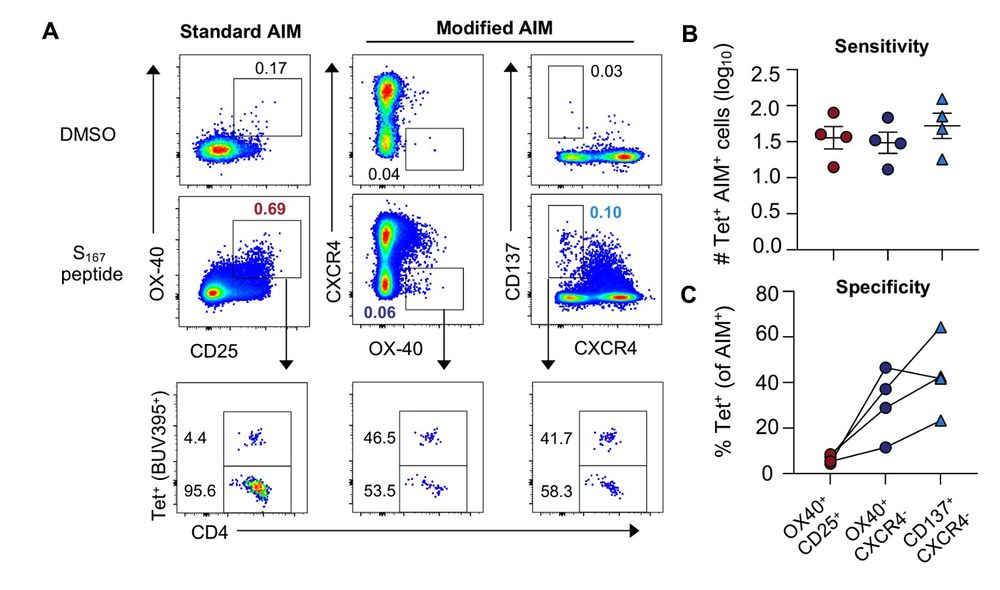
7/9 Mitch sorted tetramer-specific cells, labelled them, and added them back into PBMC before stimulating with the relevant peptide.
He was able to show that ag-specific CD4 cells downregulate CXCR4 while upregulating 4-1BB/CD137, giving us a more accurate way to define antigen specificity.
He was able to show that ag-specific CD4 cells downregulate CXCR4 while upregulating 4-1BB/CD137, giving us a more accurate way to define antigen specificity.
December 20, 2024 at 3:39 AM
7/9 Mitch sorted tetramer-specific cells, labelled them, and added them back into PBMC before stimulating with the relevant peptide.
He was able to show that ag-specific CD4 cells downregulate CXCR4 while upregulating 4-1BB/CD137, giving us a more accurate way to define antigen specificity.
He was able to show that ag-specific CD4 cells downregulate CXCR4 while upregulating 4-1BB/CD137, giving us a more accurate way to define antigen specificity.
6/9 Unfortunately, these cytokine-activated cells upregulated almost all of the AIM markers we normally use.
One difference, however, appeared to be CXCR4. TCR-mediated activation efficiently downregulates CXCR4 expression, while cytokine-mediated activation has much less of an impact.
One difference, however, appeared to be CXCR4. TCR-mediated activation efficiently downregulates CXCR4 expression, while cytokine-mediated activation has much less of an impact.
December 20, 2024 at 3:39 AM
6/9 Unfortunately, these cytokine-activated cells upregulated almost all of the AIM markers we normally use.
One difference, however, appeared to be CXCR4. TCR-mediated activation efficiently downregulates CXCR4 expression, while cytokine-mediated activation has much less of an impact.
One difference, however, appeared to be CXCR4. TCR-mediated activation efficiently downregulates CXCR4 expression, while cytokine-mediated activation has much less of an impact.
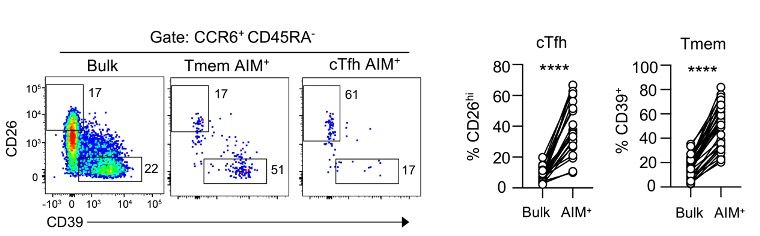
5/9 Using RNAseq and confirmatory FACS, Mitch showed that CD39+ Tregs and Th17/Th22 cells expressing CCR6 and high levels of CD26 were being activated through the transwell via a mechanism involving IL-2.
These same subsets are also found during standard peptide-based AIM assays.
These same subsets are also found during standard peptide-based AIM assays.
December 20, 2024 at 3:39 AM
5/9 Using RNAseq and confirmatory FACS, Mitch showed that CD39+ Tregs and Th17/Th22 cells expressing CCR6 and high levels of CD26 were being activated through the transwell via a mechanism involving IL-2.
These same subsets are also found during standard peptide-based AIM assays.
These same subsets are also found during standard peptide-based AIM assays.
4/9 Possibly!
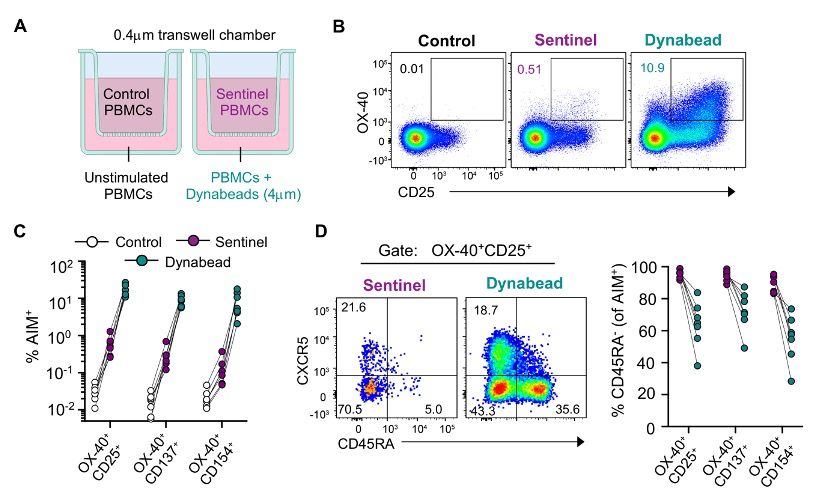
In a transwell assay, anti-CD3/CD28 stimulation can drive T cells in an adjacent chamber to express common activation markers (CD25, OX-40, 4-1BB, CD40L).
All of these responding cells had a memory phenotype, and were strongly enriched for CCR6 expression.
In a transwell assay, anti-CD3/CD28 stimulation can drive T cells in an adjacent chamber to express common activation markers (CD25, OX-40, 4-1BB, CD40L).
All of these responding cells had a memory phenotype, and were strongly enriched for CCR6 expression.
December 20, 2024 at 3:39 AM
4/9 Possibly!
In a transwell assay, anti-CD3/CD28 stimulation can drive T cells in an adjacent chamber to express common activation markers (CD25, OX-40, 4-1BB, CD40L).
All of these responding cells had a memory phenotype, and were strongly enriched for CCR6 expression.
In a transwell assay, anti-CD3/CD28 stimulation can drive T cells in an adjacent chamber to express common activation markers (CD25, OX-40, 4-1BB, CD40L).
All of these responding cells had a memory phenotype, and were strongly enriched for CCR6 expression.
3/9 Mitch ruled out a few options: CD4 cells don’t upregulate CCR6 during peptide stimulation, and the AIM+ CCR6+ cells don’t usually contain clonally expanded TCRs.
Does that mean that AIM assays might be picking up cases of TCR-independent activation?
Does that mean that AIM assays might be picking up cases of TCR-independent activation?
December 20, 2024 at 3:39 AM
3/9 Mitch ruled out a few options: CD4 cells don’t upregulate CCR6 during peptide stimulation, and the AIM+ CCR6+ cells don’t usually contain clonally expanded TCRs.
Does that mean that AIM assays might be picking up cases of TCR-independent activation?
Does that mean that AIM assays might be picking up cases of TCR-independent activation?
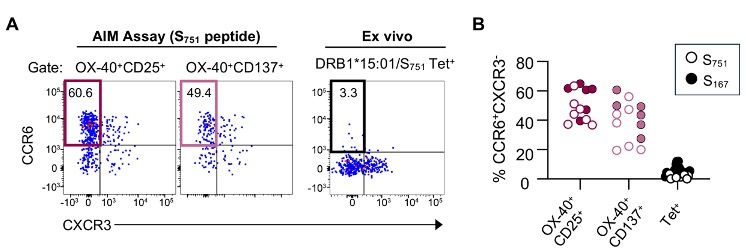
2/9 Recently, though, we’ve been bothered by something we couldn’t explain: we find a lot of Th17-like, CCR6+ SARS-CoV-2 spike-specific CD4 T cells in our AIM assays, but we never see these cells when we use spike-specific class II tetramers.
Why?
Why?
December 20, 2024 at 3:39 AM
2/9 Recently, though, we’ve been bothered by something we couldn’t explain: we find a lot of Th17-like, CCR6+ SARS-CoV-2 spike-specific CD4 T cells in our AIM assays, but we never see these cells when we use spike-specific class II tetramers.
Why?
Why?

