Dr. Jeffrey Millman
@jeffreymillman.bsky.social
Washington University School of Medicine Professor | Diabetes Researcher | Stem Cell Biologist | Bioengineer | Inventor | STEM Educator | Former MIT & Harvard | Former Biotech VP | https://sites.wustl.edu/millmanlab/
This is what I am going to get to protect my sequencing data

October 24, 2025 at 4:38 PM
This is what I am going to get to protect my sequencing data
If you would like to donate to support T1D research, please do so here: www2.breakthrought1d.org/site/TR?fr_i...

Join our Breakthrough T1D Walk team and help create a world without type 1 diabetes (T1D).
Our team is walking and raising funds to support the most promising, cutting-edge science to create a world without T1D. Join us!
www2.breakthrought1d.org
October 13, 2025 at 6:00 PM
If you would like to donate to support T1D research, please do so here: www2.breakthrought1d.org/site/TR?fr_i...
September 2, 2025 at 2:50 PM
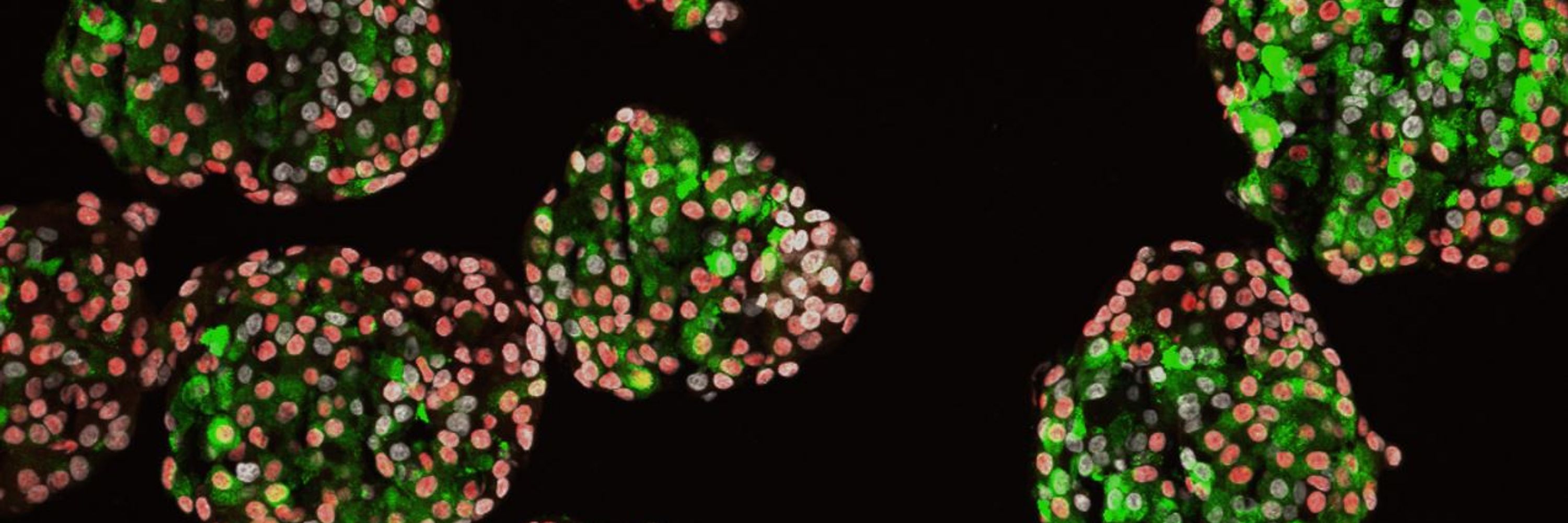
Overall, CVB3 sparks diverse, cell type-specific stress pathways in islets, with MIR7-3HG emerging as a regulator of infection + survival. These insights could help unravel viral contributions to T1D.
September 2, 2025 at 2:42 PM
Overall, CVB3 sparks diverse, cell type-specific stress pathways in islets, with MIR7-3HG emerging as a regulator of infection + survival. These insights could help unravel viral contributions to T1D.
We also identified the lncRNA MIR7-3HG as a key player. Knockdown in stem cell-derived islets ↓ viral genome levels, ↓ apoptosis, and altered autophagy, suggesting new therapeutic angles.
September 2, 2025 at 2:42 PM
We also identified the lncRNA MIR7-3HG as a key player. Knockdown in stem cell-derived islets ↓ viral genome levels, ↓ apoptosis, and altered autophagy, suggesting new therapeutic angles.
CVB3 disrupted mitochondria differently across cell types. Beta cells had increased mitochondrial size but lose efficiency. Alpha cells had shrunken mitochondria. These are potentially protective acclimations.
September 2, 2025 at 2:42 PM
CVB3 disrupted mitochondria differently across cell types. Beta cells had increased mitochondrial size but lose efficiency. Alpha cells had shrunken mitochondria. These are potentially protective acclimations.
Beta, alpha, and duct cells had the strongest responses. Beta and alpha cells demonstrated mitochondrial dysfunction. Duct had the strongest interferon & HLA responses. All cells had unique stress and immune signatures.
September 2, 2025 at 2:42 PM
Beta, alpha, and duct cells had the strongest responses. Beta and alpha cells demonstrated mitochondrial dysfunction. Duct had the strongest interferon & HLA responses. All cells had unique stress and immune signatures.
It has been a decade filled with challenges, breakthroughs, and the joy of discovery.
I cannot wait to see what the next ten years bring for my lab, my trainees, and the field of SC-islets.
Here’s to the next chapter. 🙌
I cannot wait to see what the next ten years bring for my lab, my trainees, and the field of SC-islets.
Here’s to the next chapter. 🙌
August 11, 2025 at 2:22 PM
It has been a decade filled with challenges, breakthroughs, and the joy of discovery.
I cannot wait to see what the next ten years bring for my lab, my trainees, and the field of SC-islets.
Here’s to the next chapter. 🙌
I cannot wait to see what the next ten years bring for my lab, my trainees, and the field of SC-islets.
Here’s to the next chapter. 🙌
I’m continually inspired by my interactions with people living with diabetes. Hearing your experiences reminds my team and me why our work matters and fuels our commitment to advancing SC-islet research. Your stories are the heart behind the science. Thank you!
August 11, 2025 at 2:22 PM
I’m continually inspired by my interactions with people living with diabetes. Hearing your experiences reminds my team and me why our work matters and fuels our commitment to advancing SC-islet research. Your stories are the heart behind the science. Thank you!
I’m deeply grateful to WashU Medicine and the Division of Endocrinology for fostering an environment where collaboration and innovation thrive.
Thanks also to @breakthrought1dhq.bsky.social, NIH NIDDK, and generous philanthropic donors for supporting our work.
Thanks also to @breakthrought1dhq.bsky.social, NIH NIDDK, and generous philanthropic donors for supporting our work.
August 11, 2025 at 2:22 PM
I’m deeply grateful to WashU Medicine and the Division of Endocrinology for fostering an environment where collaboration and innovation thrive.
Thanks also to @breakthrought1dhq.bsky.social, NIH NIDDK, and generous philanthropic donors for supporting our work.
Thanks also to @breakthrought1dhq.bsky.social, NIH NIDDK, and generous philanthropic donors for supporting our work.
None of this would have been possible without the people in my lab, 41 trainees so far. Mentoring them has been a privilege, and many have gone on to accomplish great things in academia and industry. Their success is one of the most rewarding parts of my career.
August 11, 2025 at 2:22 PM
None of this would have been possible without the people in my lab, 41 trainees so far. Mentoring them has been a privilege, and many have gone on to accomplish great things in academia and industry. Their success is one of the most rewarding parts of my career.
SC-islets are now widely used around the world to study human development, model disease, and advance cell replacement therapies.
It has been remarkable to see this area of research explode since we reported them in 2014 to become a cornerstone of diabetes science.
It has been remarkable to see this area of research explode since we reported them in 2014 to become a cornerstone of diabetes science.
August 11, 2025 at 2:22 PM
SC-islets are now widely used around the world to study human development, model disease, and advance cell replacement therapies.
It has been remarkable to see this area of research explode since we reported them in 2014 to become a cornerstone of diabetes science.
It has been remarkable to see this area of research explode since we reported them in 2014 to become a cornerstone of diabetes science.
The field has also made huge leaps this past decade.
Vertex reported groundbreaking clinical trial results with SC-islets, and Sana Biotechnology has successes with hypoimmune islets, showing potential for cell therapy without chronic immunosuppression.
Vertex reported groundbreaking clinical trial results with SC-islets, and Sana Biotechnology has successes with hypoimmune islets, showing potential for cell therapy without chronic immunosuppression.
August 11, 2025 at 2:22 PM
The field has also made huge leaps this past decade.
Vertex reported groundbreaking clinical trial results with SC-islets, and Sana Biotechnology has successes with hypoimmune islets, showing potential for cell therapy without chronic immunosuppression.
Vertex reported groundbreaking clinical trial results with SC-islets, and Sana Biotechnology has successes with hypoimmune islets, showing potential for cell therapy without chronic immunosuppression.
Along the way, we developed new genetic and microenvironmental models to study pancreatic islet biology and uncovered how stem cells decide to become islets and revealed new aspects of the disease state of beta cells, guiding future therapeutic strategies.
August 11, 2025 at 2:22 PM
Along the way, we developed new genetic and microenvironmental models to study pancreatic islet biology and uncovered how stem cells decide to become islets and revealed new aspects of the disease state of beta cells, guiding future therapeutic strategies.
We generated SC-islets from patients with T1D, enabling patient-specific disease modeling.
We also used gene editing to repair the Wolfram syndrome mutation in patient-derived SC-islets, a step toward precision medicine in rare forms of diabetes.
We also used gene editing to repair the Wolfram syndrome mutation in patient-derived SC-islets, a step toward precision medicine in rare forms of diabetes.
August 11, 2025 at 2:22 PM
We generated SC-islets from patients with T1D, enabling patient-specific disease modeling.
We also used gene editing to repair the Wolfram syndrome mutation in patient-derived SC-islets, a step toward precision medicine in rare forms of diabetes.
We also used gene editing to repair the Wolfram syndrome mutation in patient-derived SC-islets, a step toward precision medicine in rare forms of diabetes.

