Clinical response rates were consistent.... even for those with 4+ prior AT: 55% clinical response
In JAK-IR: 55% clinical response

Clinical response rates were consistent.... even for those with 4+ prior AT: 55% clinical response
In JAK-IR: 55% clinical response
Obefazimod works in patients with prior AT-IR, perhaps not as well as in AT-naive patients.

Obefazimod works in patients with prior AT-IR, perhaps not as well as in AT-naive patients.
Obefazimod was generally well-tolerated. Treatment-Emergent Adverse Events (TEAEs) and Serious Adverse Events (SAEs) were comparable across all three groups (Placebo, 25mg, 50mg). Headaches did not result in treatment cessation.

Obefazimod was generally well-tolerated. Treatment-Emergent Adverse Events (TEAEs) and Serious Adverse Events (SAEs) were comparable across all three groups (Placebo, 25mg, 50mg). Headaches did not result in treatment cessation.
Both Obefazimod doses achieved clinically meaningful improvements across ALL efficacy endpoints in the pooled dataset. The (higher) 50mg dose showed superior outcomes.

Both Obefazimod doses achieved clinically meaningful improvements across ALL efficacy endpoints in the pooled dataset. The (higher) 50mg dose showed superior outcomes.
Notably, the study included a highly refractory population, with ~21% having prior AT-IR (inadequate response) failing a JAK inhibitor.

Notably, the study included a highly refractory population, with ~21% having prior AT-IR (inadequate response) failing a JAK inhibitor.
The ABTECT Phase 3 program includes 2 induction trials & 1 maintenance trial. Our focus today is on the strong safety and efficacy results from the ABTECT 1 & 2 induction phases.

The ABTECT Phase 3 program includes 2 induction trials & 1 maintenance trial. Our focus today is on the strong safety and efficacy results from the ABTECT 1 & 2 induction phases.
- week 8 induction data presented at #UEGWeek
- first in class for IBD
1/🧵
MoA: ⬆️expression of miR-124, which regulates inflammatory response & restores mucosal homeostasis.

- week 8 induction data presented at #UEGWeek
- first in class for IBD
1/🧵
MoA: ⬆️expression of miR-124, which regulates inflammatory response & restores mucosal homeostasis.
63% in clinical remission…
But only 32% in transmural remission on IUS
➡️ Almost HALF of “well” patients still had active disease

63% in clinical remission…
But only 32% in transmural remission on IUS
➡️ Almost HALF of “well” patients still had active disease
No prep 🛑
No radiation ☢️
Point-of-care, takes minutes ⏱️
Sees transmural inflammation, not just mucosa
Minimal carbon footprint ♻️




No prep 🛑
No radiation ☢️
Point-of-care, takes minutes ⏱️
Sees transmural inflammation, not just mucosa
Minimal carbon footprint ♻️
1/ #IBD is rising fast in SE Asia 📈
We need tools that are accurate, patient-friendly, and sustainable.
Our SGH pilot study shows #IntestinalUltrasound ticks all 3 boxes ✅

1/ #IBD is rising fast in SE Asia 📈
We need tools that are accurate, patient-friendly, and sustainable.
Our SGH pilot study shows #IntestinalUltrasound ticks all 3 boxes ✅
- ↓ hsCRP & fecal calprotectin by Week 6
- ↓ Th1, Th17 cytokines (IL-17A, IL-22, IL-9)
- ↓ fibrotic gene expression (e.g., COL1A1, MMP3)
- Histologic remission (RHI <3) in 18%


- ↓ hsCRP & fecal calprotectin by Week 6
- ↓ Th1, Th17 cytokines (IL-17A, IL-22, IL-9)
- ↓ fibrotic gene expression (e.g., COL1A1, MMP3)
- Histologic remission (RHI <3) in 18%
- AEs: 78% (mostly mild-moderate)
- SAEs: 15% (none drug-related)
- No deaths, no infusion reactions
- Most common AE: infection (45%), incl. COVID-19 (11%)

- AEs: 78% (mostly mild-moderate)
- SAEs: 15% (none drug-related)
- No deaths, no infusion reactions
- Most common AE: infection (45%), incl. COVID-19 (11%)
- Endoscopic response (≥50% ↓ in SES-CD): 26.0%
vs historical placebo 12% (p=0.0023)
- Clinical remission (CDAI <150): 49.1%
vs historical placebo 16% (p<0.0001)


- Endoscopic response (≥50% ↓ in SES-CD): 26.0%
vs historical placebo 12% (p=0.0023)
- Clinical remission (CDAI <150): 49.1%
vs historical placebo 16% (p<0.0001)
- Multicentre, open-label
- Moderate-to-severe CD
- IV tulisokibart: 1000 mg (Day 1), 500 mg (Weeks 2, 6, 10)
- Endoscopic response at Week 12 = primary endpoint
71% had prior biologic exposure; 35% had ≥3 agents


- Multicentre, open-label
- Moderate-to-severe CD
- IV tulisokibart: 1000 mg (Day 1), 500 mg (Weeks 2, 6, 10)
- Endoscopic response at Week 12 = primary endpoint
71% had prior biologic exposure; 35% had ≥3 agents
Crohn's: journals.lww.com/ajg/fulltext...
UC: journals.lww.com/ajg/fulltext...
#IBDSky #MedSky #GISky




Crohn's: journals.lww.com/ajg/fulltext...
UC: journals.lww.com/ajg/fulltext...
#IBDSky #MedSky #GISky
- Any clinical remission: 37%
- Steroid-free remission: only 16%
- 66% were still on therapy at 12 months.
🛑 ICI was stopped in 80% due to colitis — but discontinuation did NOT impact remission (P=0.48).

- Any clinical remission: 37%
- Steroid-free remission: only 16%
- 66% were still on therapy at 12 months.
🛑 ICI was stopped in 80% due to colitis — but discontinuation did NOT impact remission (P=0.48).
CRP? Mildly elevated.
➡️ Don’t underestimate colitis just because systemic markers look ‘okay’.

CRP? Mildly elevated.
➡️ Don’t underestimate colitis just because systemic markers look ‘okay’.
- Lung (42.7%)
- Melanoma (31.2%)
📆 Median time to colitis onset: 4 months after starting ICI.
💩 100% had diarrhoea, often moderate to severe.

- Lung (42.7%)
- Melanoma (31.2%)
📆 Median time to colitis onset: 4 months after starting ICI.
💩 100% had diarrhoea, often moderate to severe.
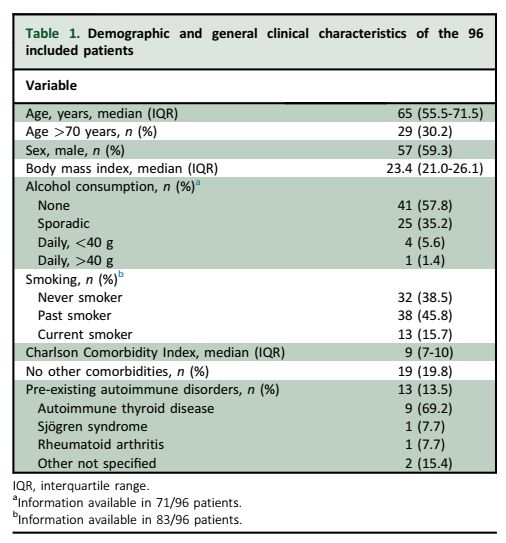
1/ ICI-induced colitis
📈 Affects up to 30% of patients on ICIs.
🔬 This study followed 96 patients with 12-month outcomes.
www.esmoopen.com/article/S205...
1/ ICI-induced colitis
📈 Affects up to 30% of patients on ICIs.
🔬 This study followed 96 patients with 12-month outcomes.
www.esmoopen.com/article/S205...