
leveraging self-assembly on electrified interfaces for catalysis ⚡
Sign up for our info sessions on Nov. 4 and Nov. 11th - anyone who registers and attends will get an application fee waiver!
chemistry.uchicago.edu/graduate-pro...

Sign up for our info sessions on Nov. 4 and Nov. 11th - anyone who registers and attends will get an application fee waiver!
chemistry.uchicago.edu/graduate-pro...
We are super excited about our vibrant research community, and you could be a part of it! Applications are also now open!
chemistry.uchicago.edu/graduate-pro...
We are super excited about our vibrant research community, and you could be a part of it! Applications are also now open!
chemistry.uchicago.edu/graduate-pro...
Our electrode-orthogonal self-assembled layers can "self-heal" after degradation -- the key is a dynamic linkage we can molecularly tune! Hats off to Nico!
pubs.acs.org/doi/full/10....

Our electrode-orthogonal self-assembled layers can "self-heal" after degradation -- the key is a dynamic linkage we can molecularly tune! Hats off to Nico!
pubs.acs.org/doi/full/10....
pubs.acs.org/doi/full/10....
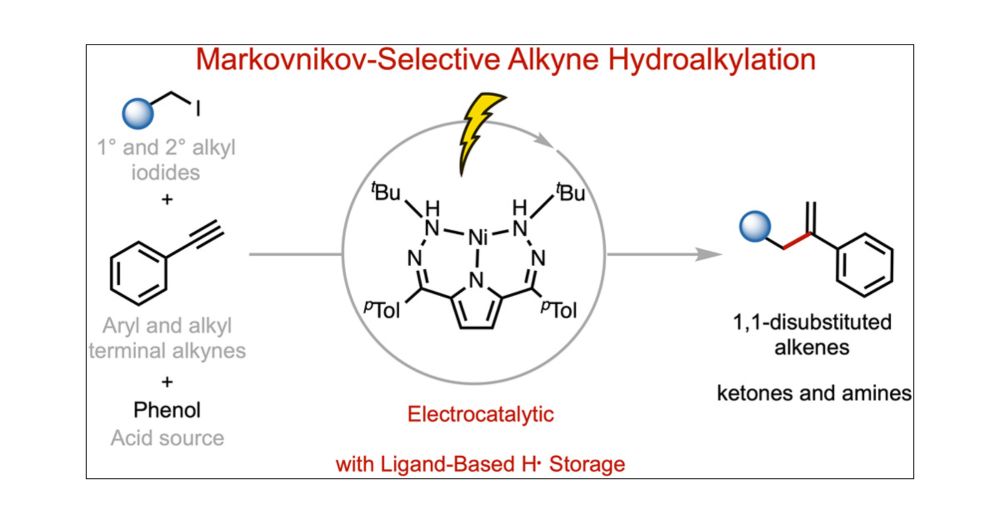
pubs.acs.org/doi/full/10....
Disorder activates graphitic carbon for echem rxns—but can we distinguish it in situ? Varying disorder shows different electric-field IR responses!
w/ Ry, Ben & calculations from Arpan in Galli Group
onlinelibrary.wiley.com/doi/10.1002/...
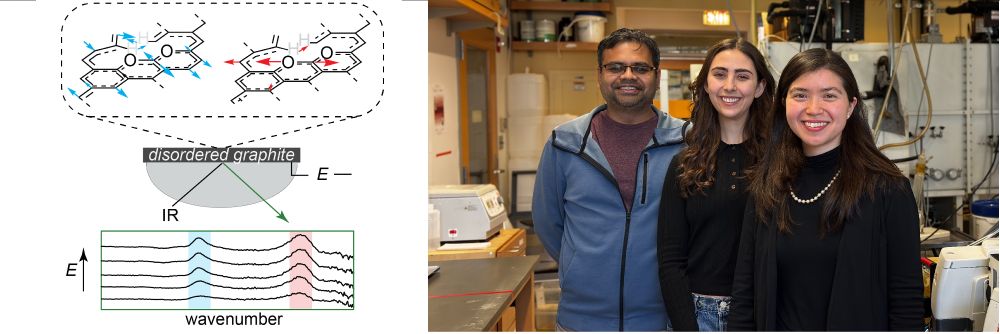
Disorder activates graphitic carbon for echem rxns—but can we distinguish it in situ? Varying disorder shows different electric-field IR responses!
w/ Ry, Ben & calculations from Arpan in Galli Group
onlinelibrary.wiley.com/doi/10.1002/...
pubs.rsc.org/en/content/a...
pubs.rsc.org/en/content/a...
Prospective students, especially those interested in the application process and/or fee waivers are encouraged to attend!
Prospective students, especially those interested in the application process and/or fee waivers are encouraged to attend!

