
Like to write a thread? Please DM us!
💬 @onenimesa.bsky.social & @juliaeckert.bsky.social
👉 https://epithelialmechanics.github.io
buff.ly/4AWd5Hk

buff.ly/4AWd5Hk
buff.ly/oGeaTex

buff.ly/oGeaTex
buff.ly/61xmO7O

buff.ly/61xmO7O
buff.ly/61xmO7O

buff.ly/61xmO7O
buff.ly/a2lGTYz

buff.ly/a2lGTYz
buff.ly/p7NvbM3

buff.ly/p7NvbM3
I’ll take you through how cells in a tissue can use information distributed by biochemical gradients to make decisions, and how we can measure such positional information.
buff.ly/RFxVeHh

I’ll take you through how cells in a tissue can use information distributed by biochemical gradients to make decisions, and how we can measure such positional information.
buff.ly/RFxVeHh





buff.ly/cByoHep
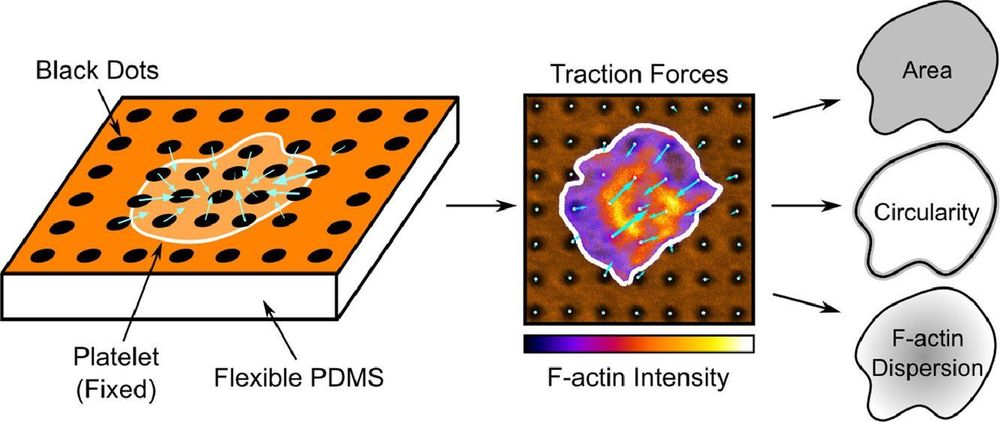
buff.ly/cByoHep
buff.ly/S91NL9V

buff.ly/S91NL9V
buff.ly/hBnA91z

buff.ly/hBnA91z
buff.ly/vyjt4nk

buff.ly/vyjt4nk
bsky.app/profile/epim...

bsky.app/profile/epim...
bsky.app/profile/epim...

bsky.app/profile/epim...
bsky.app/profile/epim...
bsky.app/profile/epim...
bsky.app/profile/epim...
bsky.app/profile/epim...
bsky.app/profile/epim...
bsky.app/profile/epim...

bsky.app/profile/epim...
bsky.app/profile/epim...
bsky.app/profile/epim...
bsky.app/profile/epim...
I’m @alex-plum.bsky.social (@mattiaserra.bsky.social group) and I’ll be sharing some papers on characterizing and controlling avian gastrulation flows.
I’m @alex-plum.bsky.social (@mattiaserra.bsky.social group) and I’ll be sharing some papers on characterizing and controlling avian gastrulation flows.

