
Deputy manager of @cirm-fungi.bsky.social Biological Resource Centre (BRC) CIRM-CF https://www.cirm-fungi.fr




So glad and honored our lab BBF (@inrae.bsky.social, @amu.bsky.social) contributed to the study of this new class of enzymes !
Congrats to the whole team, especially to first author Clelton A. Santos and to project leader Mario Murakami. www.nature.com/articles/s41...
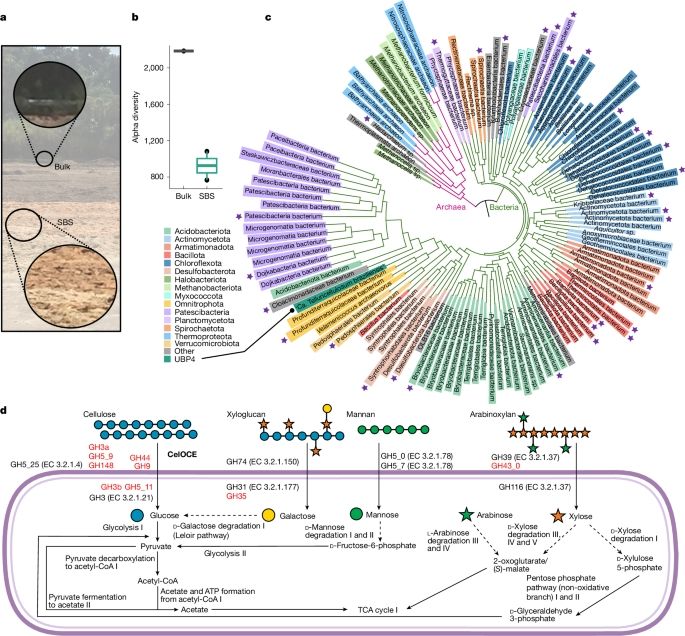
So glad and honored our lab BBF (@inrae.bsky.social, @amu.bsky.social) contributed to the study of this new class of enzymes !
Congrats to the whole team, especially to first author Clelton A. Santos and to project leader Mario Murakami. www.nature.com/articles/s41...
INRAE vous présente ses meilleurs vœux et vous souhaite une belle et heureuse année 2025

INRAE vous présente ses meilleurs vœux et vous souhaite une belle et heureuse année 2025
pubs.acs.org/doi/10.1021/...
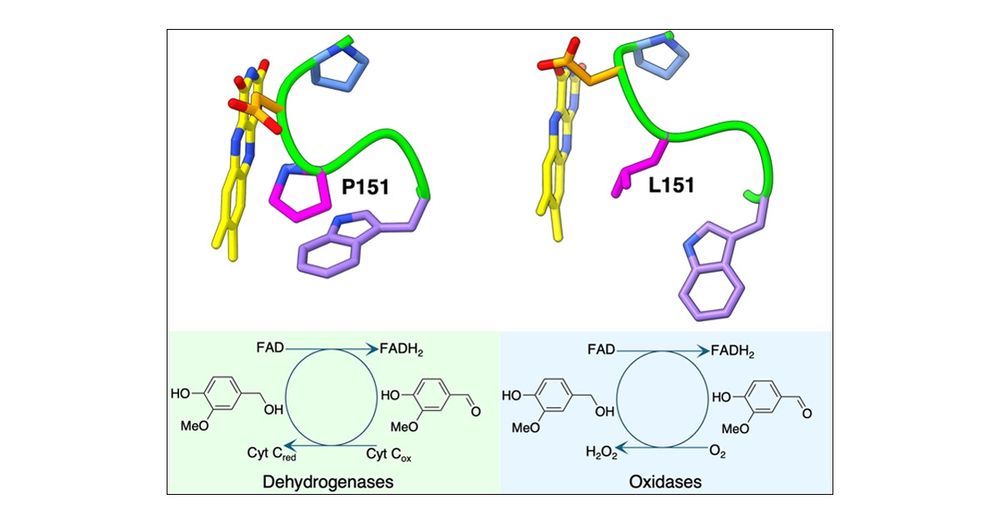
pubs.acs.org/doi/10.1021/...
🌐🍁🧪

@inrae-france.bsky.social @univ-amu.fr


@inrae-france.bsky.social @univ-amu.fr


