Lab: https://cbi-toulouse.fr/eng/equipe-krndija
Personal: https://cbi-toulouse.fr/eng/page-personnelle-49
The junctional response is therefore essential for maintaining epithelial integrity (10/11)

The junctional response is therefore essential for maintaining epithelial integrity (10/11)
👉 Mechanosensitive Ca²⁺ influx is both necessary and sufficient for junctional reinforcement under force ⚡️(9/11)

👉 Mechanosensitive Ca²⁺ influx is both necessary and sufficient for junctional reinforcement under force ⚡️(9/11)
Blocking mechanosensitive ion channels (with Gd³⁺) or chelating extracellular Ca²⁺ (with BAPTA) prevented NMII activation and junctional reinforcement – stopping the mechano-adaptive response in its tracks (8/11)

Blocking mechanosensitive ion channels (with Gd³⁺) or chelating extracellular Ca²⁺ (with BAPTA) prevented NMII activation and junctional reinforcement – stopping the mechano-adaptive response in its tracks (8/11)
👉 NMII acts as a central effector coordinating force sensing and junctional reinforcement.🔩 (7/11)

👉 NMII acts as a central effector coordinating force sensing and junctional reinforcement.🔩 (7/11)
Unexpectedly, myosin IIC, usually linked to microvilli, also relocalized to junctions under force ⚙️ (6/11)

Unexpectedly, myosin IIC, usually linked to microvilli, also relocalized to junctions under force ⚙️ (6/11)
- Tight & adherens junctions → sustained reinforcement
- Desmosomes & keratin filaments → progressive accumulation over time (5/11)

- Tight & adherens junctions → sustained reinforcement
- Desmosomes & keratin filaments → progressive accumulation over time (5/11)
The adult gut epithelium actively adapts to physiological mechanical stress 💪 (4/11)

The adult gut epithelium actively adapts to physiological mechanical stress 💪 (4/11)
Most studies remove luminal contents before analysis – we didn’t.
Keeping them revealed that the colonic mucosa adapts to faecal distension through large-scale tissue unfolding and epithelial deformation (3/11)

Most studies remove luminal contents before analysis – we didn’t.
Keeping them revealed that the colonic mucosa adapts to faecal distension through large-scale tissue unfolding and epithelial deformation (3/11)

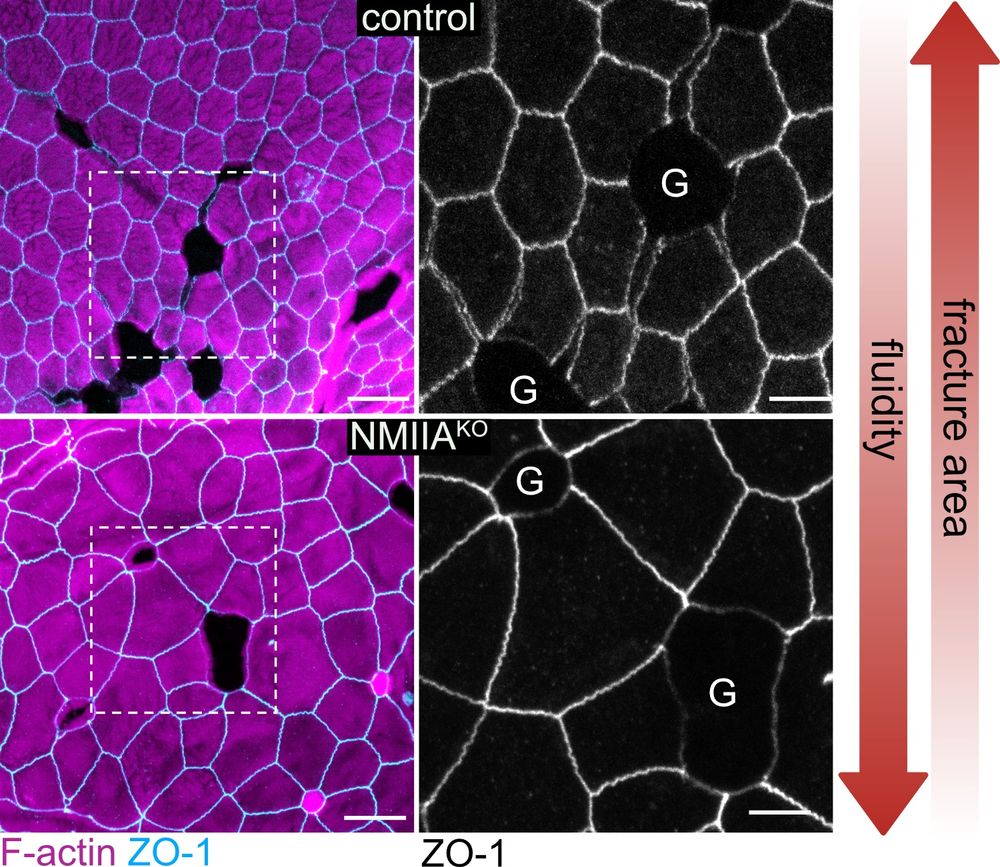
1) Goblet size/pressure → bigger goblets = more GAFs
2) Tissue fluidity → more fluid = fewer GAFs! (7/9)
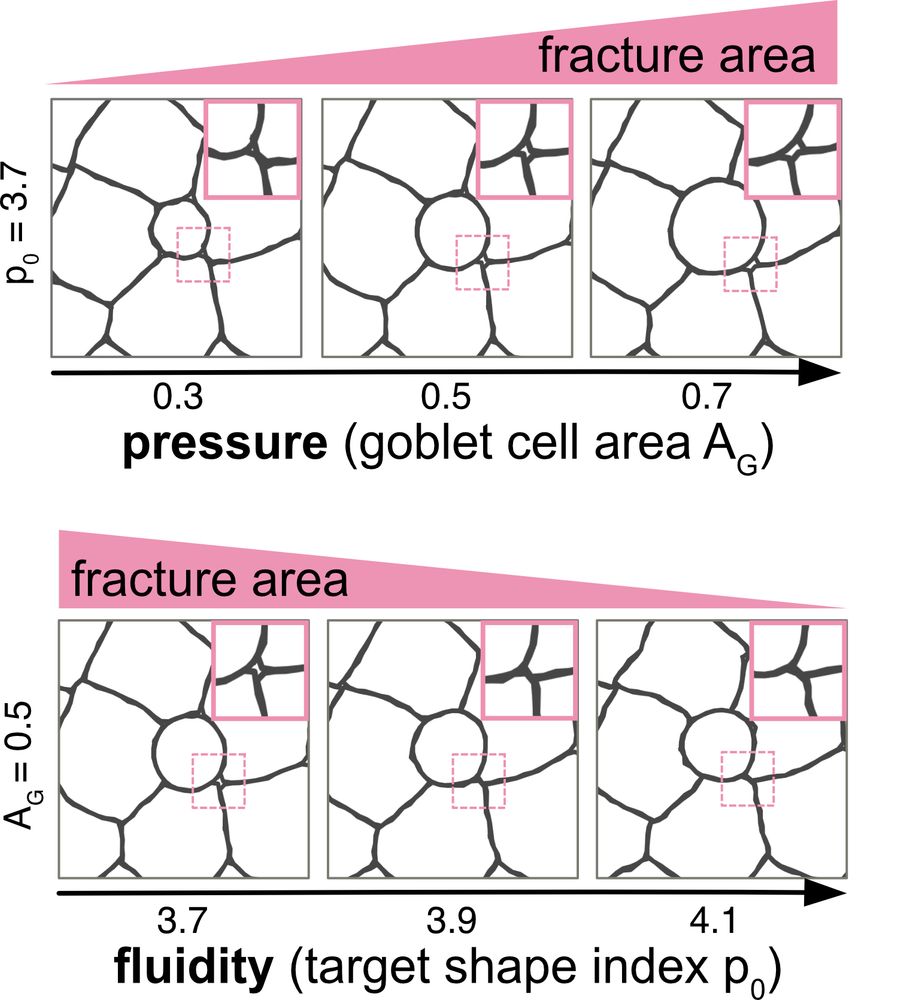
1) Goblet size/pressure → bigger goblets = more GAFs
2) Tissue fluidity → more fluid = fewer GAFs! (7/9)
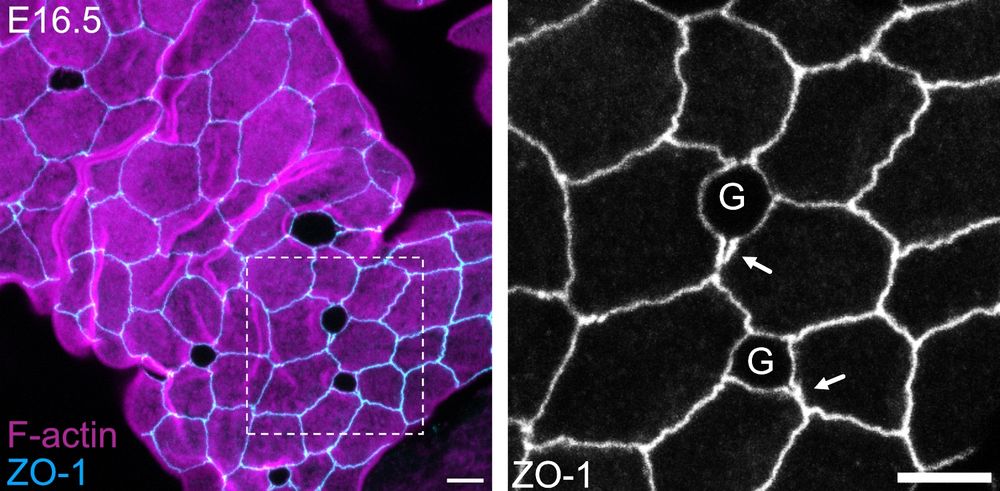
No! They accumulate E-cadherin & other junctional proteins in a mechano-sensitive response to goblet-induced strain! Yet, goblet hypertrophy overcomes this active response, leading to full junctional failure in a tight > adherens > desmosome hierarchy! ⚠️ (4/9)
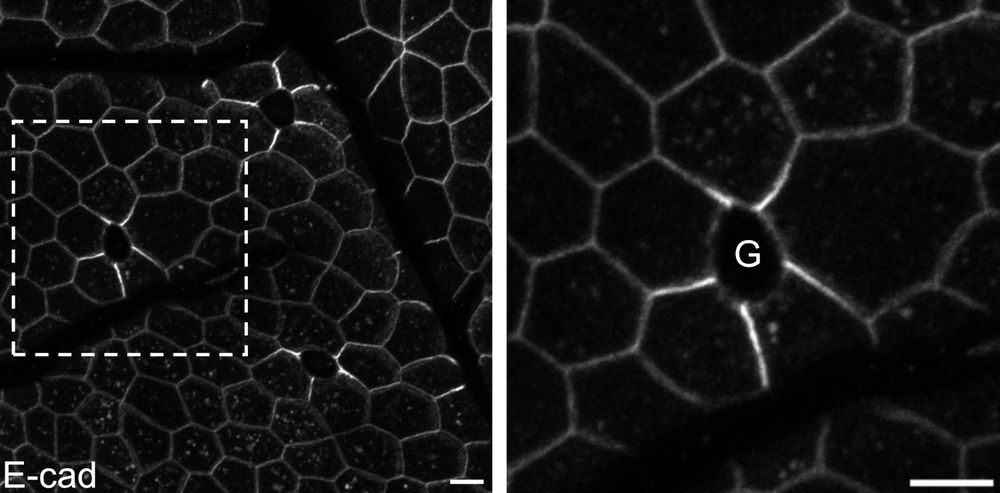
No! They accumulate E-cadherin & other junctional proteins in a mechano-sensitive response to goblet-induced strain! Yet, goblet hypertrophy overcomes this active response, leading to full junctional failure in a tight > adherens > desmosome hierarchy! ⚠️ (4/9)
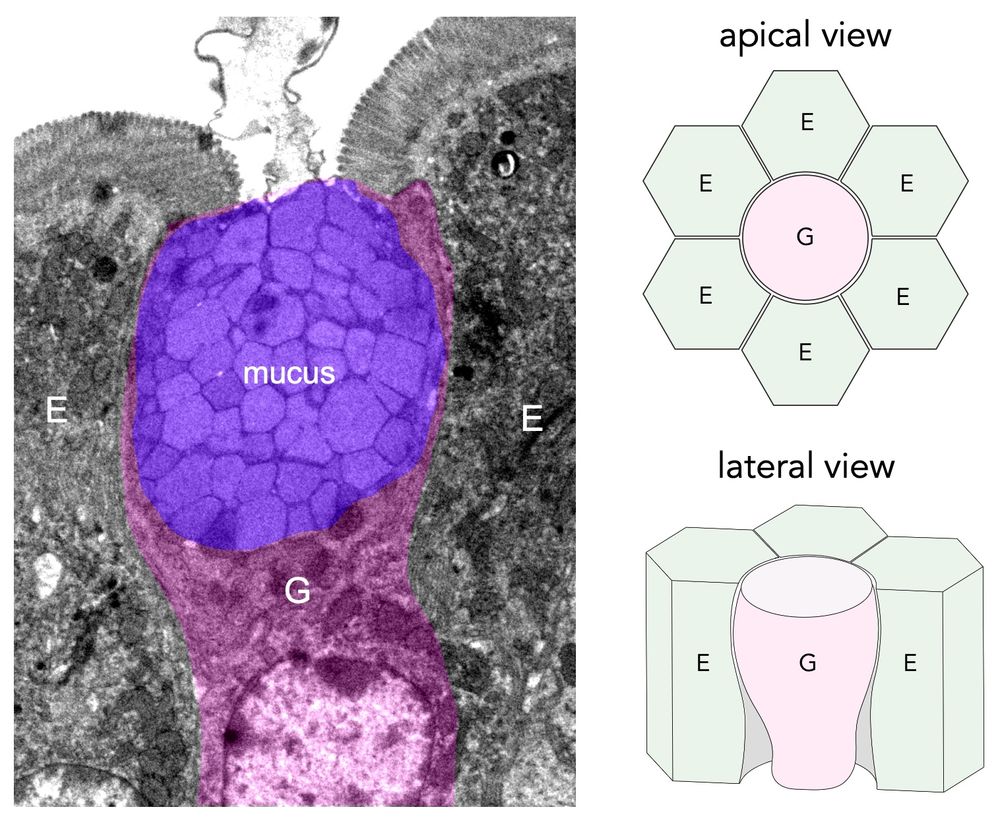
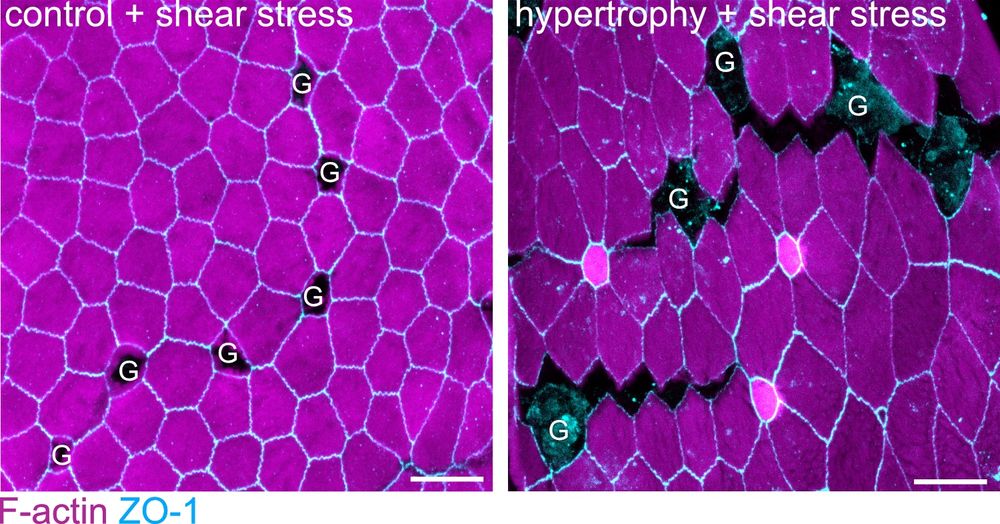
They compress & deform neighboring enterocytes, straining their junctions to the point of rupture! GAFs are regulated by goblet cell volume – goblet hypertrophy, often a protective response (parasite clearance), paradoxically increases fracturing & permeability! 🤯 (3/9)
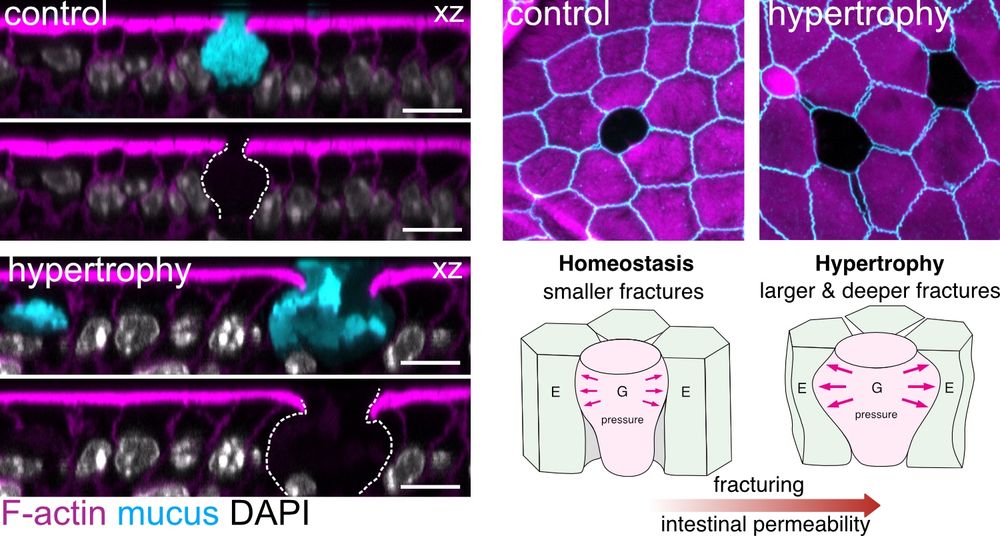
They compress & deform neighboring enterocytes, straining their junctions to the point of rupture! GAFs are regulated by goblet cell volume – goblet hypertrophy, often a protective response (parasite clearance), paradoxically increases fracturing & permeability! 🤯 (3/9)