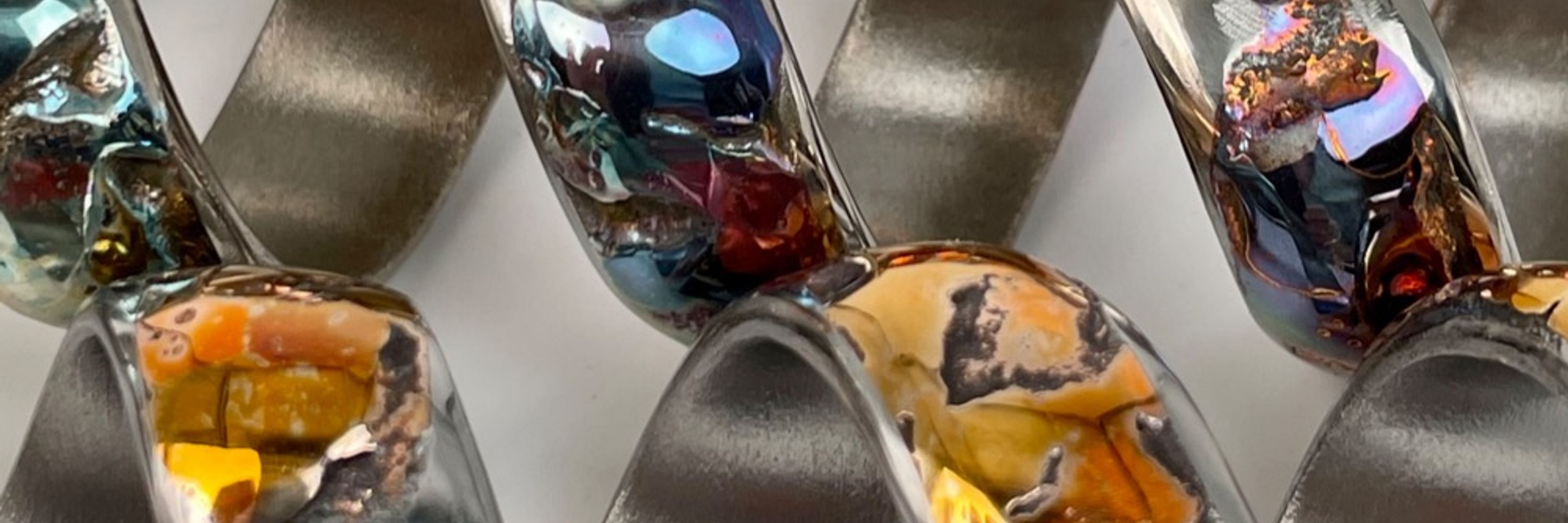
Bryan Welm
@bryanwelm.bsky.social
Science artist and cancer researcher. Huntsman Cancer Institute, University of Utah
www.bryanwelm.com
www.bryanwelm.com
That’s what I think. It has to do with the depth of the oxide within the copper, which is controlled by the amount of heat. Wavelengths of light reflect off different oxide layers and can interfere with each other, resulting in subtraction of some wavelengths/colors.
December 25, 2024 at 7:15 PM
That’s what I think. It has to do with the depth of the oxide within the copper, which is controlled by the amount of heat. Wavelengths of light reflect off different oxide layers and can interfere with each other, resulting in subtraction of some wavelengths/colors.
got some answers. 1. metalworking shop; 2. I braze/weld copper for some sculptures. Sometimes the copper gets so hot a molten drop falls and goes splat on the floor; 3. The different oxidation states might be from how quickly they cool eg. if they land on the metal welding table vs the floor.
December 25, 2024 at 1:30 AM
got some answers. 1. metalworking shop; 2. I braze/weld copper for some sculptures. Sometimes the copper gets so hot a molten drop falls and goes splat on the floor; 3. The different oxidation states might be from how quickly they cool eg. if they land on the metal welding table vs the floor.
DM and we can chat.
December 20, 2024 at 1:57 AM
DM and we can chat.

