Beck Laboratory
@becklab.bsky.social
Dpt. for #MolecularSociology @mpibp.bsky.social
https://www.biophys.mpg.de/molecular-sociology
https://www.biophys.mpg.de/molecular-sociology
(3/3) We propose a model, where the NPC dynamically acts to buffer tension applied to the nuclear envelope, thereby protecting it from potential rupture. Aberrations of the NPC scaffold would cause a reduced buffering capacity and result in NPC disintegration and potential rupture.

April 11, 2025 at 1:26 PM
(3/3) We propose a model, where the NPC dynamically acts to buffer tension applied to the nuclear envelope, thereby protecting it from potential rupture. Aberrations of the NPC scaffold would cause a reduced buffering capacity and result in NPC disintegration and potential rupture.
(2/3) Nup133 is a conserved component of the Y-complex, its deletion however is surprisingly is not lethal, but causes tissue and cell-type specific defects. We found that NPCs in Nup133-/- cells over-stretch and disintegrate upon induction of neuronal differentiation.

April 11, 2025 at 1:26 PM
(2/3) Nup133 is a conserved component of the Y-complex, its deletion however is surprisingly is not lethal, but causes tissue and cell-type specific defects. We found that NPCs in Nup133-/- cells over-stretch and disintegrate upon induction of neuronal differentiation.
(1/3) Together with the labs of Valerie Doye @vdoye.bsky.social and Hans-Georg-Kräusslich, we analyzed NPC structure in Nup133 null mESCs. We found heterogenous NPCs featuring non-canonical symmetries and missing subunits. Now online @naturecellbiology.bsky.social.
www.nature.com/articles/s41...
www.nature.com/articles/s41...

April 11, 2025 at 1:26 PM
(1/3) Together with the labs of Valerie Doye @vdoye.bsky.social and Hans-Georg-Kräusslich, we analyzed NPC structure in Nup133 null mESCs. We found heterogenous NPCs featuring non-canonical symmetries and missing subunits. Now online @naturecellbiology.bsky.social.
www.nature.com/articles/s41...
www.nature.com/articles/s41...
Summarized, our data suggest a HIV-1 nuclear entry model where first a CypA coat around the capsid gets stripped away while the capsid is drawn into the FG-Nup mesh of NPC. Then the compression of the FG-Nups forces the NPC scaffold to crack allowing further progression of the capsid.

January 17, 2025 at 6:43 PM
Summarized, our data suggest a HIV-1 nuclear entry model where first a CypA coat around the capsid gets stripped away while the capsid is drawn into the FG-Nup mesh of NPC. Then the compression of the FG-Nups forces the NPC scaffold to crack allowing further progression of the capsid.
Through MD simulations we show that capsids face a significant steric barrier inside the NPC, especially when the vast amount of intrinsically disordered FG-Nups in the central channel are included. This barrier could be relieved by a crack in the NPC.
January 17, 2025 at 6:43 PM
Through MD simulations we show that capsids face a significant steric barrier inside the NPC, especially when the vast amount of intrinsically disordered FG-Nups in the central channel are included. This barrier could be relieved by a crack in the NPC.
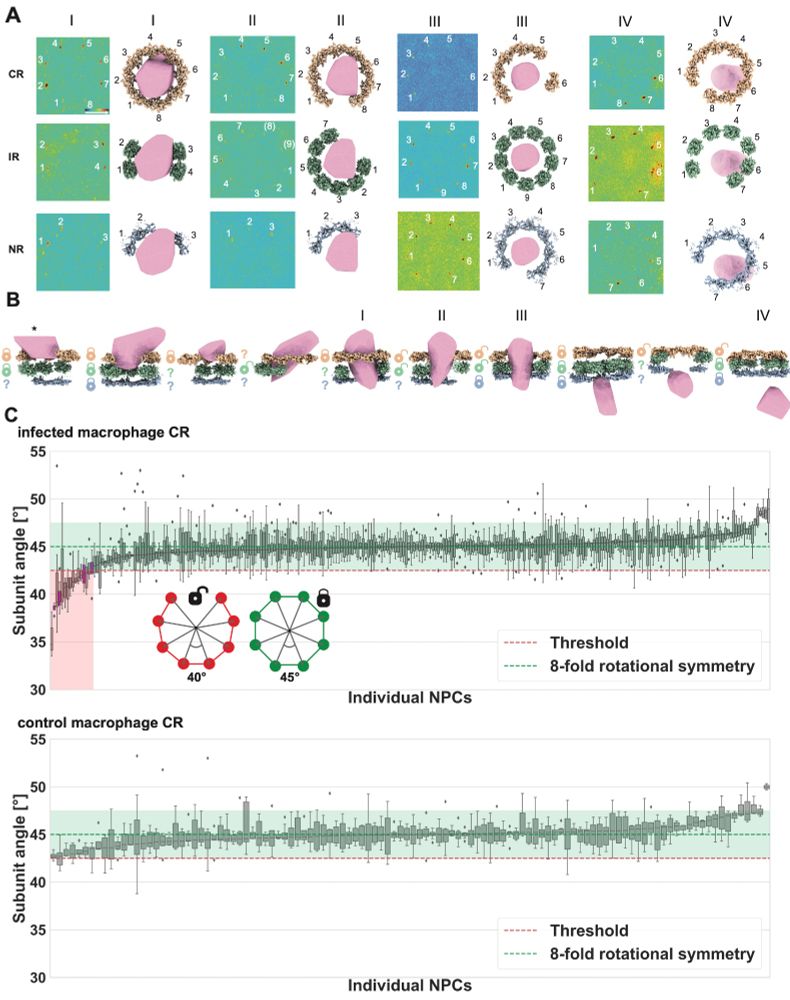
Through template matching NPC ring subunits, we were able to investigate structural changes to individual NPCs and found that capsid presence is significantly linked to NPCs being cracked open, meaning the subunit geometry exceeds the normal 8-fold rotational symmetry in the NPC scaffold.
January 17, 2025 at 6:43 PM
Through template matching NPC ring subunits, we were able to investigate structural changes to individual NPCs and found that capsid presence is significantly linked to NPCs being cracked open, meaning the subunit geometry exceeds the normal 8-fold rotational symmetry in the NPC scaffold.
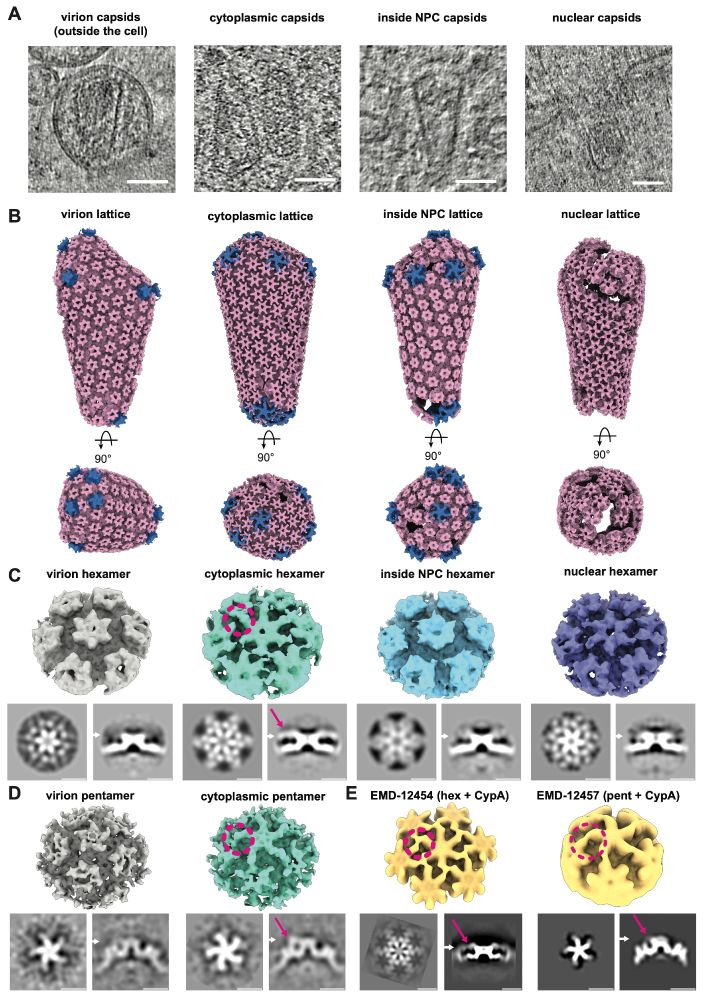
Using cryo-ET subtomogram averaging (#TeamTomo), we traced capsid (CA) lattices in virions, the cytoplasm, NPC, and nucleus. Cytoplasmic CA hexamers and pentamers showed bound density consistent with CypA, lost upon NPC entry. The CypA coat likely protects the capsid from host restriction factors.
January 17, 2025 at 6:43 PM
Using cryo-ET subtomogram averaging (#TeamTomo), we traced capsid (CA) lattices in virions, the cytoplasm, NPC, and nucleus. Cytoplasmic CA hexamers and pentamers showed bound density consistent with CypA, lost upon NPC entry. The CypA coat likely protects the capsid from host restriction factors.
CLEM-ET data showed that morphologically intact cone-shaped capsids could be found inside the central channel of NPCs and also directly underneath in the nucleus further solidifying that capsids do not need to disassemble for nuclear entry.
January 17, 2025 at 6:43 PM
CLEM-ET data showed that morphologically intact cone-shaped capsids could be found inside the central channel of NPCs and also directly underneath in the nucleus further solidifying that capsids do not need to disassemble for nuclear entry.
The HIV capsids were detected on the cytoplasmic side, inside the central channel, and on the nuclear side of the NPCs. These data suggest that passage through the NPC is a significant hurdle for the capsid.

January 17, 2025 at 6:43 PM
The HIV capsids were detected on the cytoplasmic side, inside the central channel, and on the nuclear side of the NPCs. These data suggest that passage through the NPC is a significant hurdle for the capsid.
@jpkreysing.bsky.social, @maziarheidari.bsky.social, Vojtech Zila, and others studied HIV-1 capsid nuclear entry in primary human macrophages using STED, CLEM-ET, cryo-ET (#TeamTomo), and MD simulations. First, 3D STED revealed capsid signal accumulation at nuclear pores.
January 17, 2025 at 6:43 PM
@jpkreysing.bsky.social, @maziarheidari.bsky.social, Vojtech Zila, and others studied HIV-1 capsid nuclear entry in primary human macrophages using STED, CLEM-ET, cryo-ET (#TeamTomo), and MD simulations. First, 3D STED revealed capsid signal accumulation at nuclear pores.
In a great collaboration with @hummerlab.bsky.social and the Kräusslich lab: HIV capsid doesn't break at the NPC; instead, it cracks open the NPC itself! Details in Cell: authors.elsevier.com/sd/article/S... @mpibp.bsky.social @uniheidelberg.bsky.social A thread below:
January 17, 2025 at 6:43 PM
In a great collaboration with @hummerlab.bsky.social and the Kräusslich lab: HIV capsid doesn't break at the NPC; instead, it cracks open the NPC itself! Details in Cell: authors.elsevier.com/sd/article/S... @mpibp.bsky.social @uniheidelberg.bsky.social A thread below:

