

#photochemistry #CPA #deracemization
doi.org/10.1039/D5SC...

#photochemistry #CPA #deracemization
doi.org/10.1039/D5SC...
'Photocatalytic reductive incorporation of carbon dioxide into double bonds' 🔓
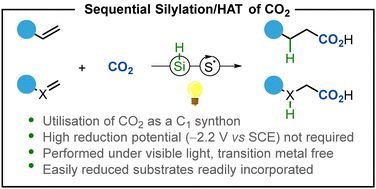
'Photocatalytic reductive incorporation of carbon dioxide into double bonds' 🔓
doi.org/10.1021/jacs.5c07524
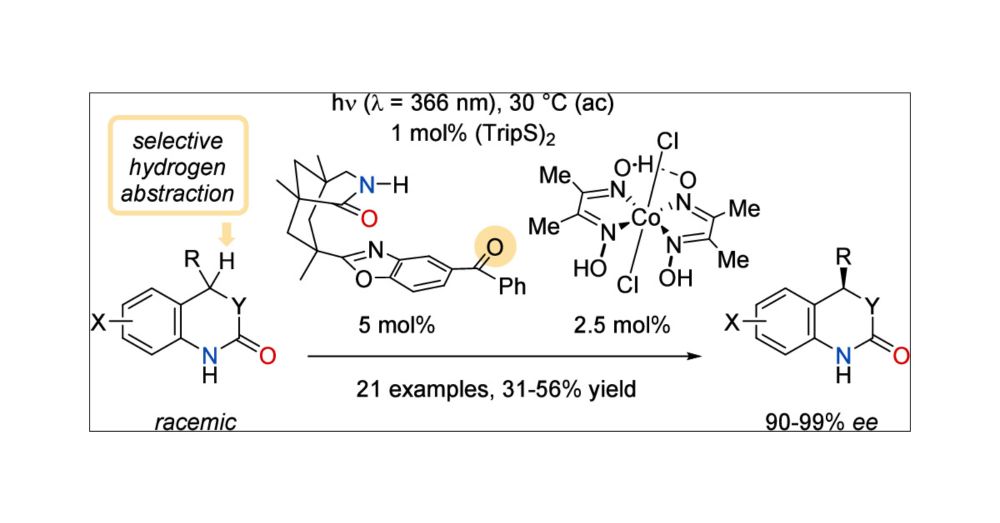
doi.org/10.1021/jacs.5c07524
So proud of everyone who took part! Huge participation and great energy all around.
Special shoutout to Simone for finishing 3rd fastest woman over 11 km! 🔥
Big thanks to our awesome cheer squad 🫶 You really made a difference! 💙💪
#CampusRun #Race #TUM


So proud of everyone who took part! Huge participation and great energy all around.
Special shoutout to Simone for finishing 3rd fastest woman over 11 km! 🔥
Big thanks to our awesome cheer squad 🫶 You really made a difference! 💙💪
#CampusRun #Race #TUM
www.nature.com/articles/s41...
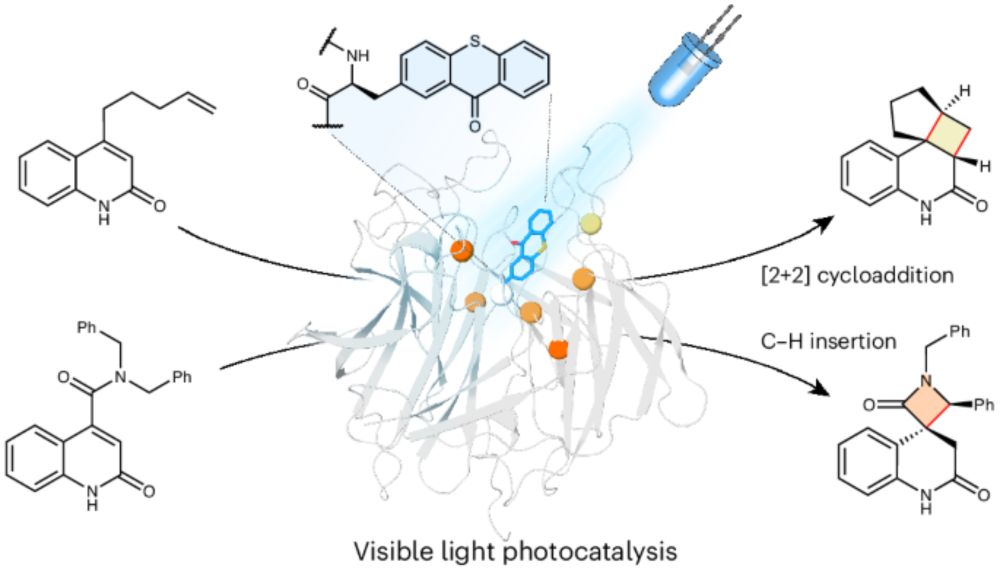
www.nature.com/articles/s41...

doi.org/10.1021/jacs...
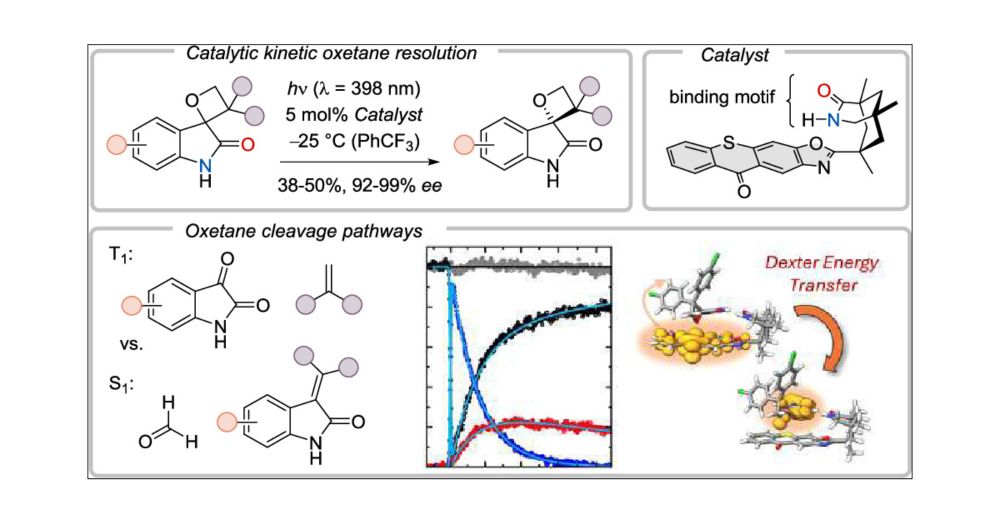
doi.org/10.1021/jacs...



pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...
onlinelibrary.wiley.com/doi/10.1002/...

onlinelibrary.wiley.com/doi/10.1002/...

pubs.acs.org/doi/full/10....
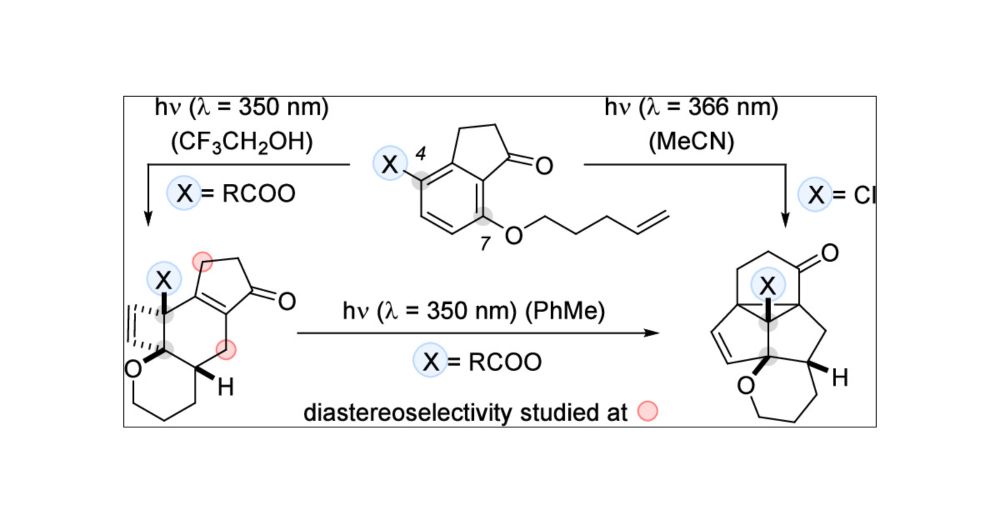
pubs.acs.org/doi/full/10....



pubs.acs.org/doi/10.1021/...
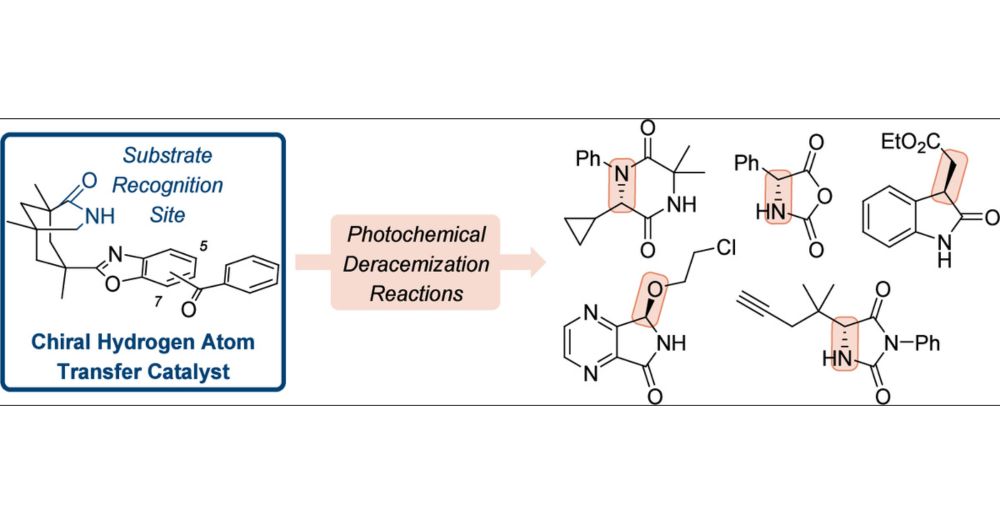
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/jacs.4c16053
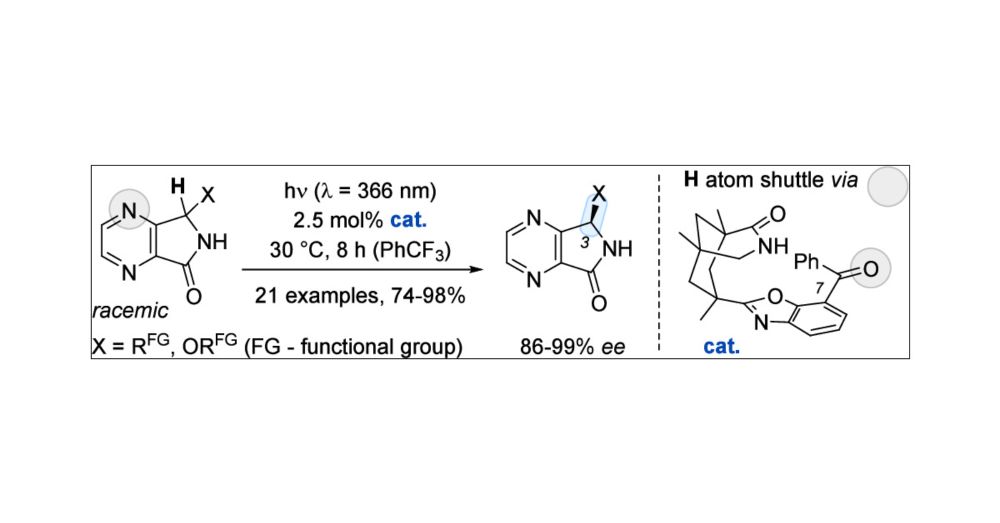
pubs.acs.org/doi/10.1021/jacs.4c16053
ch.nat.tum.de/oc1

ch.nat.tum.de/oc1






