Above 0 degrees Celsius, this CO complex rearranges to form a tin carbene complex.
🔗 CSD Entry ELAMIR: dx.doi.org/10.5517/ccdc...
#FeaturedStructureFriday #CompChemSky
Above 0 degrees Celsius, this CO complex rearranges to form a tin carbene complex.
🔗 CSD Entry ELAMIR: dx.doi.org/10.5517/ccdc...
#FeaturedStructureFriday #CompChemSky
www.science.org/doi/10.1126/...
@humboldt-foundation.de
@oxfordchemistry.bsky.social
@ox.ac.uk

www.science.org/doi/10.1126/...
@humboldt-foundation.de
@oxfordchemistry.bsky.social
@ox.ac.uk
Check out the work from the Holger Braunschweig lab, here:
pubs.rsc.org/en/content/a...
📍 @uni-wuerzburg.de
👨👩👧👦 @maxdietz.bsky.social, Merle Arrowsmith, Holger Braunschweig
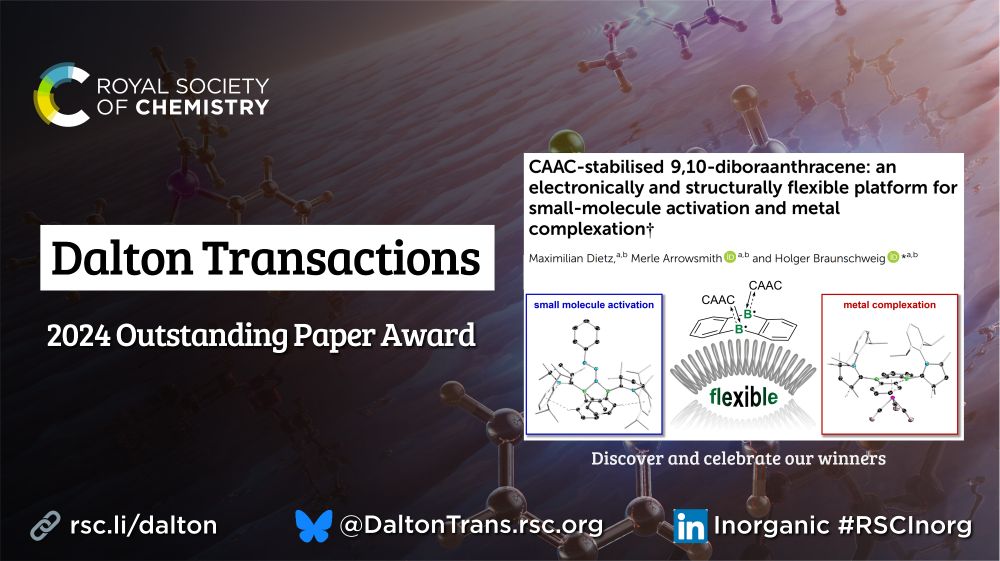
Check out the work from the Holger Braunschweig lab, here:
pubs.rsc.org/en/content/a...
📍 @uni-wuerzburg.de
👨👩👧👦 @maxdietz.bsky.social, Merle Arrowsmith, Holger Braunschweig
@maxdietz.bsky.social gave a talk, and the rest of the group presented posters.


@maxdietz.bsky.social gave a talk, and the rest of the group presented posters.
Great to be a part of this paper with @maxdietz.bsky.social and @josefboronski.bsky.social investigating some exciting tin chemistry.
@angewandtechemie.bsky.social @maxdietz.bsky.social @amelia-swarbrook.bsky.social
doi.org/10.1002/anie...

Great to be a part of this paper with @maxdietz.bsky.social and @josefboronski.bsky.social investigating some exciting tin chemistry.
pubs.acs.org/doi/10.1021/...
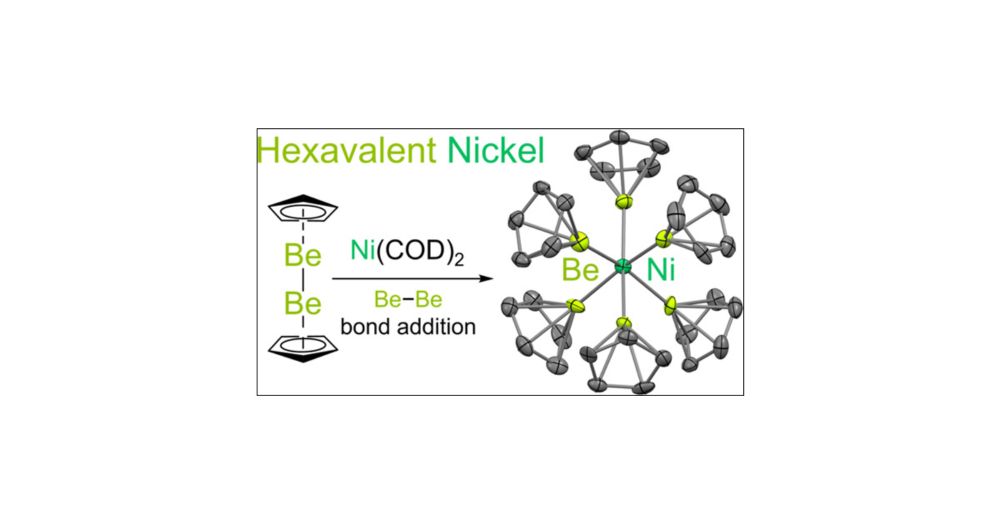
pubs.acs.org/doi/10.1021/...

