
INSERM investigator. Group leader @institut_curie Paris.
(We can change our citation of your work from BioRxiv to Nature now ;))
(We can change our citation of your work from BioRxiv to Nature now ;))
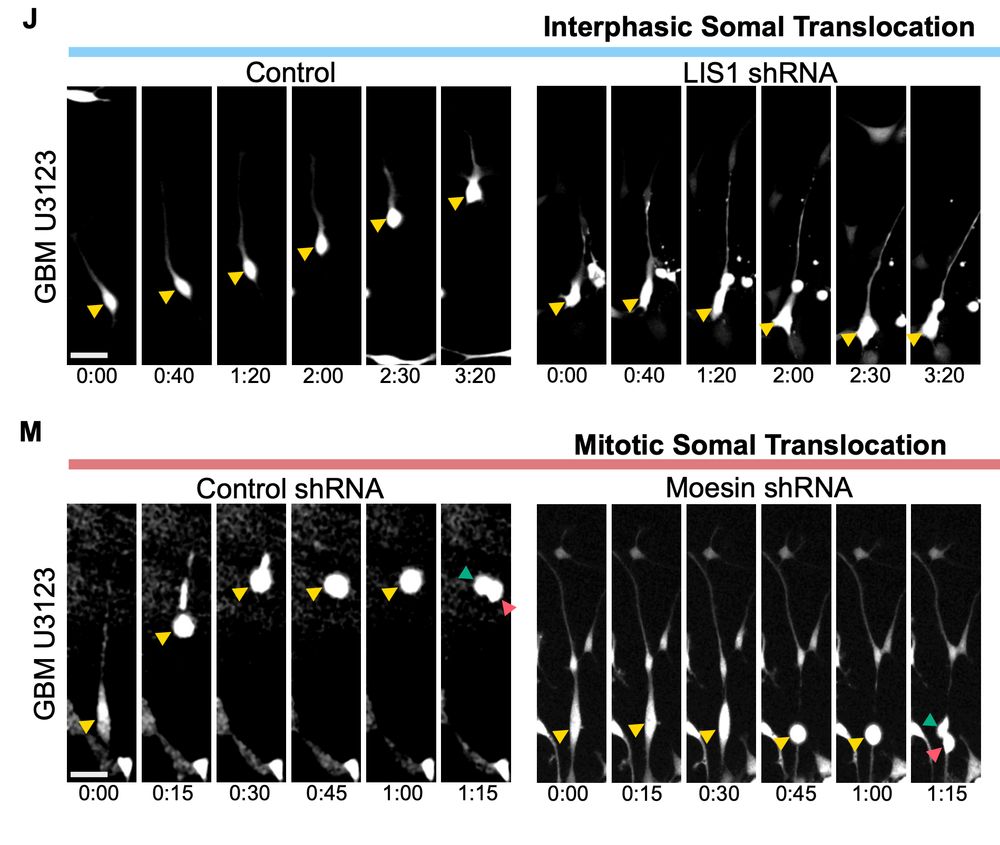
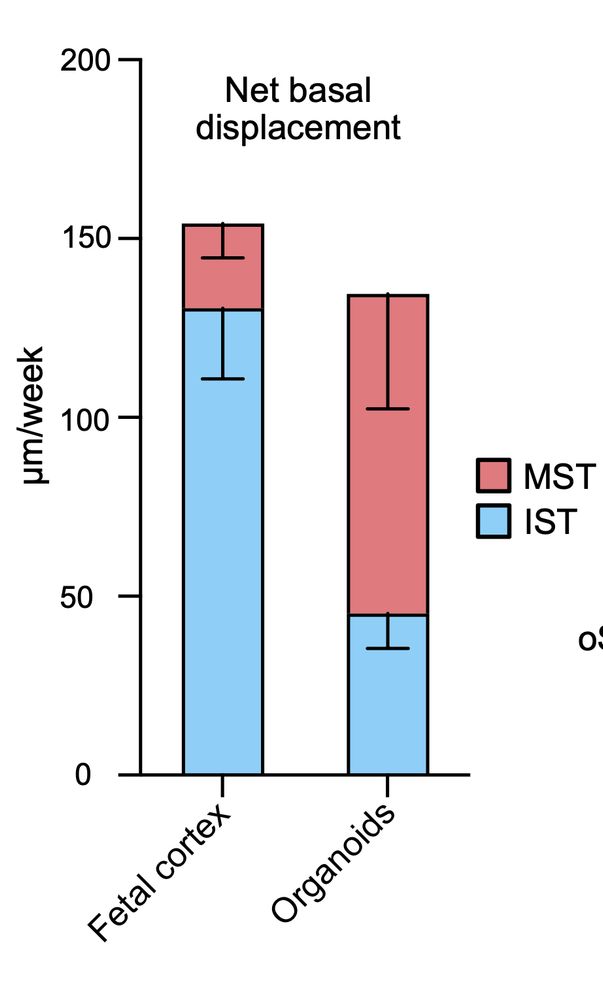
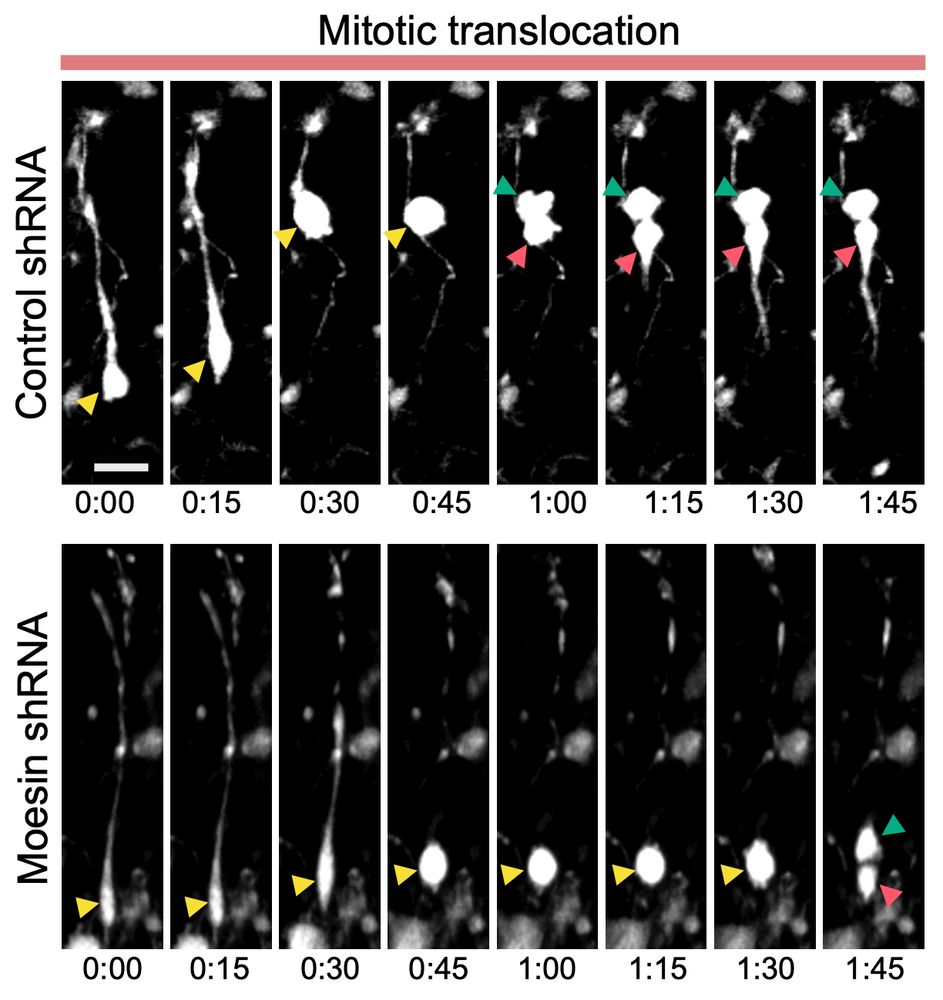
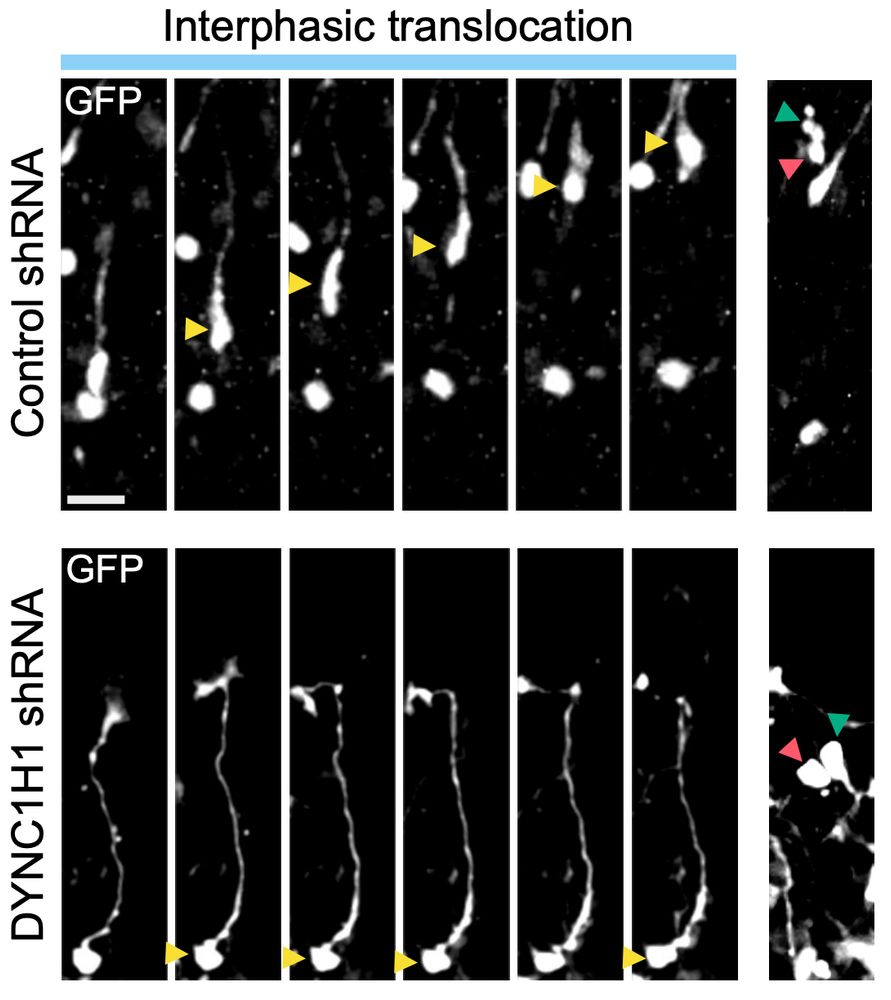
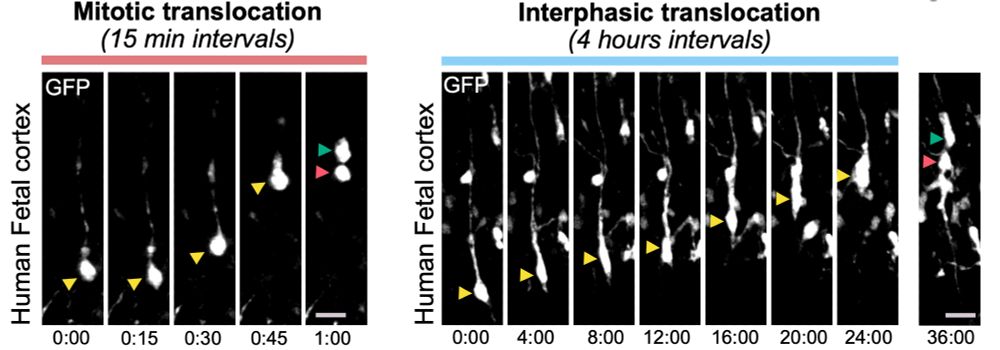
What he realised is that bRG cells have two, totally independent modes of migration, for which he managed to identify the molecular mechanisms.
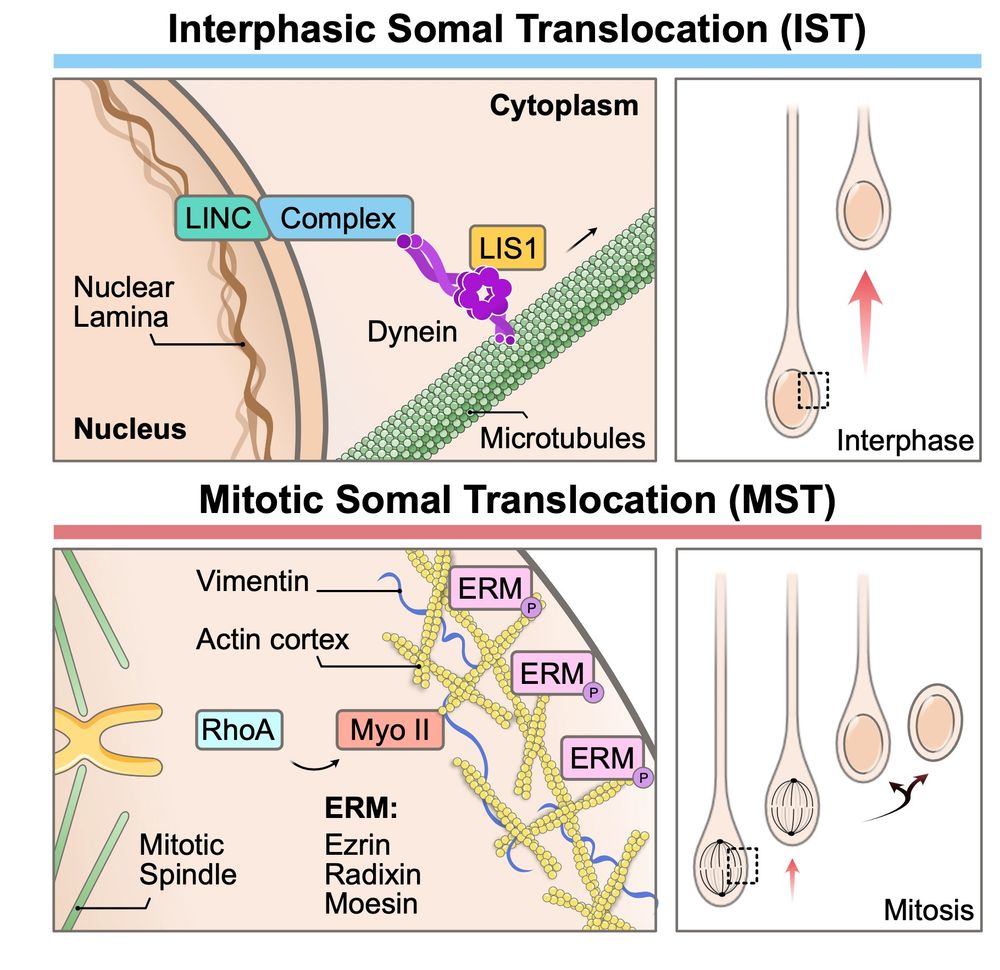
What he realised is that bRG cells have two, totally independent modes of migration, for which he managed to identify the molecular mechanisms.

