Now it just asks if the patient was assigned Male or Female at birth.
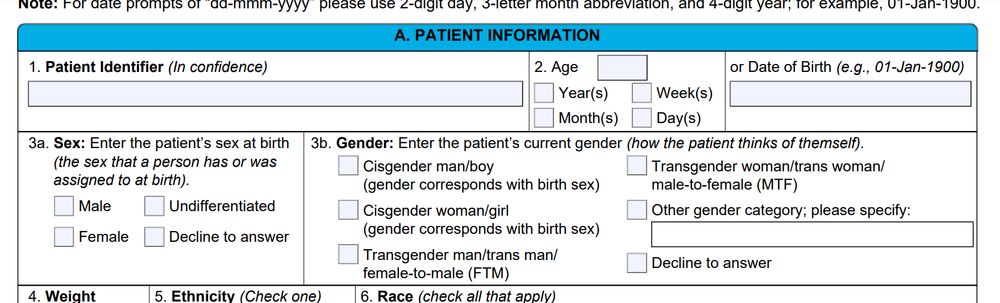
Now it just asks if the patient was assigned Male or Female at birth.
One of the wildest warning letters I've ever read:
www.fda.gov/inspections-...

One of the wildest warning letters I've ever read:
www.fda.gov/inspections-...
time.com/7201565/pers...

time.com/7201565/pers...
This piece is considerably more nuanced than any other I have seen to date:
home.agencyiq.com/article/0000...

This piece is considerably more nuanced than any other I have seen to date:
home.agencyiq.com/article/0000...
Guidance documents are non-binding documents commonly used by FDA to guide the development of regulated products.
If SCOTUS says FDA can't change its mind, FDA might shy away from them.


Guidance documents are non-binding documents commonly used by FDA to guide the development of regulated products.
If SCOTUS says FDA can't change its mind, FDA might shy away from them.
I mean, who among us doesn't hide batch production records on the roof during an FDA inspection?
www.fda.gov/inspections-...

I mean, who among us doesn't hide batch production records on the roof during an FDA inspection?
www.fda.gov/inspections-...
This lede from POLITICO's @danielpayne.bsky.social really captures a lot of the caution right now among advocacy groups, industry groups and lobbyists:
www.politico.com/news/2024/11...


This lede from POLITICO's @danielpayne.bsky.social really captures a lot of the caution right now among advocacy groups, industry groups and lobbyists:
www.politico.com/news/2024/11...
But not everyone. My POLITICO colleague @meganmesserly.bsky.social reports that some Dem-aligned groups are assembling a "war room."
www.politico.com/live-updates...

But not everyone. My POLITICO colleague @meganmesserly.bsky.social reports that some Dem-aligned groups are assembling a "war room."
www.politico.com/live-updates...
A fascinating find here from my POLITICO colleague Carmen Paun:
www.politico.com/newsletters/...

A fascinating find here from my POLITICO colleague Carmen Paun:
www.politico.com/newsletters/...




As my analysis shows, there are some major risks for the FDA and life sciences industry:
home.agencyiq.com/article/0000...

As my analysis shows, there are some major risks for the FDA and life sciences industry:
home.agencyiq.com/article/0000...
I went back through AgencyIQ's archives to find examples of cases where that happened, and... it happened quite a bit during Trump's last term.
www.agencyiq.com/blog/the-tru...

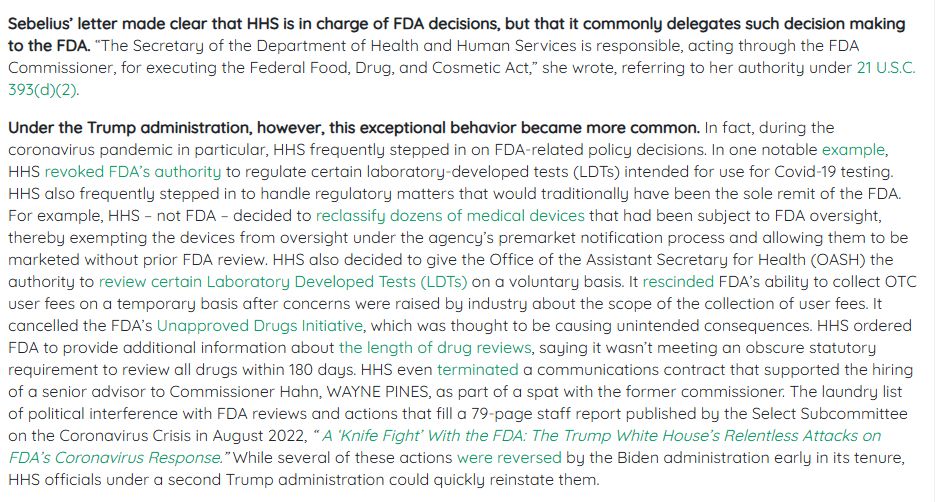
I went back through AgencyIQ's archives to find examples of cases where that happened, and... it happened quite a bit during Trump's last term.
www.agencyiq.com/blog/the-tru...

(This is from FDA's publicly available FOIA logs from 2023)

(This is from FDA's publicly available FOIA logs from 2023)

