
Ashley Sullivan
@aesully98.bsky.social
🦬 // PhD candidate in the Aaron Whiteley Lab at CU Boulder. she/her
Finally, thank you to @mfwhite2.bsky.social for the wonderful summary of these stories! www.nature.com/articles/d41...

Bacteria use a decoy defence molecule to set a trap for viruses
Some bacterial-infecting viruses use ‘sponges’ to mop up defence molecules, but bacteria can fight back by responding when a sponge captures decoy molecules.
www.nature.com
October 3, 2025 at 4:11 PM
Finally, thank you to @mfwhite2.bsky.social for the wonderful summary of these stories! www.nature.com/articles/d41...
Congrats as well to @erinedoherty.bsky.social and @benadler.bsky.social et al. in the @doudna-lab.bsky.social lab for their excellent complementary work on Panoptes! www.nature.com/articles/s41...

A miniature CRISPR–Cas10 enzyme confers immunity by inhibitory signalling - Nature
Panoptes, an anti-phage defence system against virus-mediated immune suppression, is revealed.
www.nature.com
October 3, 2025 at 4:11 PM
Congrats as well to @erinedoherty.bsky.social and @benadler.bsky.social et al. in the @doudna-lab.bsky.social lab for their excellent complementary work on Panoptes! www.nature.com/articles/s41...
Huge shoutout and thanks to my lab mates in the @aaronwhiteley.bsky.social lab and our collaborators in the @benmorehouse.bsky.social lab. This work would not have been possible without you all! 🫶🏻
October 3, 2025 at 4:11 PM
Huge shoutout and thanks to my lab mates in the @aaronwhiteley.bsky.social lab and our collaborators in the @benmorehouse.bsky.social lab. This work would not have been possible without you all! 🫶🏻
Reposted by Ashley Sullivan
Very excited to see our work appear alongside findings from @aaronwhiteley.bsky.social & @benmorehouse.bsky.social !
www.nature.com/articles/s41...
www.nature.com/articles/s41...

The Panoptes system uses decoy cyclic nucleotides to defend against phage - Nature
The Panoptes antiphage system defends bacteria by detecting phage-encoded counter-defences that sequester cyclic nucleotide signals, triggering membrane disruption and highlighting a broader strategy of sensing immune evasion through second-messenger surveillance.
www.nature.com
October 2, 2025 at 3:16 AM
Very excited to see our work appear alongside findings from @aaronwhiteley.bsky.social & @benmorehouse.bsky.social !
www.nature.com/articles/s41...
www.nature.com/articles/s41...
Reposted by Ashley Sullivan
Thank you to everyone who helped make this story happen!
Obligatory meme to summarize:
“How I imagine phage facing CBASS and mCpol”
Obligatory meme to summarize:
“How I imagine phage facing CBASS and mCpol”

October 1, 2025 at 5:57 PM
Thank you to everyone who helped make this story happen!
Obligatory meme to summarize:
“How I imagine phage facing CBASS and mCpol”
Obligatory meme to summarize:
“How I imagine phage facing CBASS and mCpol”
Reposted by Ashley Sullivan
Our sister-story from our friends @aesully98.bsky.social , @benmorehouse.bsky.social and @aaronwhiteley.bsky.social is out today as well! Congratulations!!
www.nature.com/articles/s41...
www.nature.com/articles/s41...

The Panoptes system uses decoy cyclic nucleotides to defend against phage - Nature
The Panoptes antiphage system defends bacteria by detecting phage-encoded counter-defences that sequester cyclic nucleotide signals, triggering membrane disruption and highlighting a broader strategy of sensing immune evasion through second-messenger surveillance.
www.nature.com
October 1, 2025 at 5:57 PM
Our sister-story from our friends @aesully98.bsky.social , @benmorehouse.bsky.social and @aaronwhiteley.bsky.social is out today as well! Congratulations!!
www.nature.com/articles/s41...
www.nature.com/articles/s41...
Also—check out the complementary work led by @erinedoherty.bsky.social and @benadler.bsky.social in @doudna-lab.bsky.social lab that just went up on bioRxiv!

A miniature CRISPR-Cas10 enzyme confers immunity by an inverse signaling pathway
Microbial and viral co-evolution has created immunity mechanisms involving oligonucleotide signaling that share mechanistic features with human anti-viral systems. In these pathways, including CBASS a...
www.biorxiv.org
March 31, 2025 at 10:00 PM
Also—check out the complementary work led by @erinedoherty.bsky.social and @benadler.bsky.social in @doudna-lab.bsky.social lab that just went up on bioRxiv!
Huge shout-out to all of the authors from the @aaronwhiteley.bsky.social and @benmorehouse.bsky.social labs that were so instrumental to this project! A special thank you to our collaborators L. Aravind and Max Burroughs for their incredible bioinformatic analyses!
March 31, 2025 at 10:00 PM
Huge shout-out to all of the authors from the @aaronwhiteley.bsky.social and @benmorehouse.bsky.social labs that were so instrumental to this project! A special thank you to our collaborators L. Aravind and Max Burroughs for their incredible bioinformatic analyses!
In total, we uncovered a novel mechanism of immune evasion sensing in bacteria. The Panoptes system is part of a “layered” immune system that flips the liability of a nucleotide-derived second messenger in immune signaling against the phage, adding an interesting dimension to phage-bacteria warfare.
March 31, 2025 at 10:00 PM
In total, we uncovered a novel mechanism of immune evasion sensing in bacteria. The Panoptes system is part of a “layered” immune system that flips the liability of a nucleotide-derived second messenger in immune signaling against the phage, adding an interesting dimension to phage-bacteria warfare.
Finally, we wanted to explore the relationship between CBASS and Panoptes, given the importance of Acb2 in both nucleotide-based signaling systems. We found that 53% of all Panoptes systems co-occur with CBASS, suggesting that Panoptes systems are predominantly guardians of CBASS in bacteria.
March 31, 2025 at 10:00 PM
Finally, we wanted to explore the relationship between CBASS and Panoptes, given the importance of Acb2 in both nucleotide-based signaling systems. We found that 53% of all Panoptes systems co-occur with CBASS, suggesting that Panoptes systems are predominantly guardians of CBASS in bacteria.
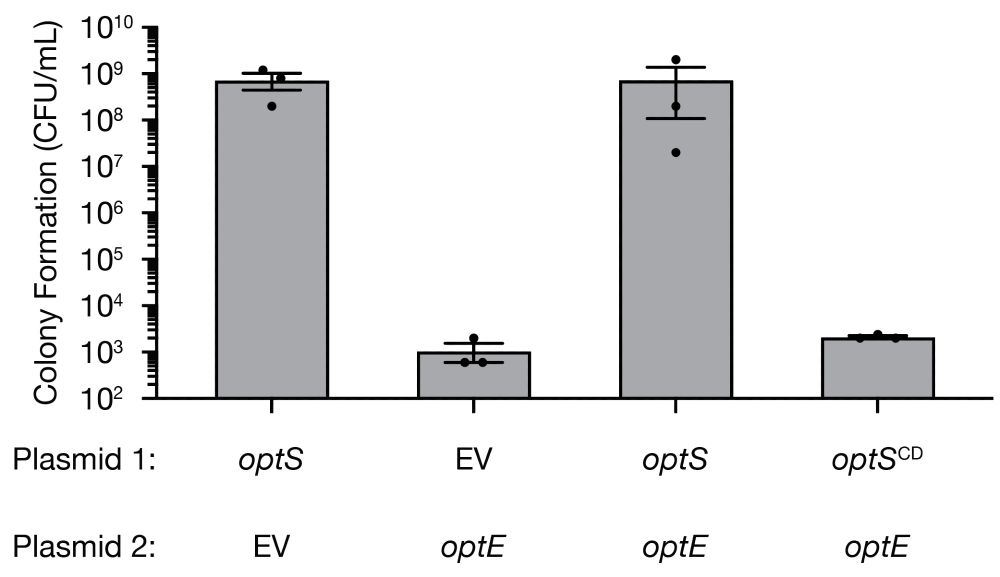
Consistent with this, we found that OptE robustly inhibited bacterial growth unless an OptS-derived nucleotide was present and that OptS constitutively synthesizes 2′,3′-c-di-AMP in vivo in the absence of phage infection.
March 31, 2025 at 10:00 PM
Consistent with this, we found that OptE robustly inhibited bacterial growth unless an OptS-derived nucleotide was present and that OptS constitutively synthesizes 2′,3′-c-di-AMP in vivo in the absence of phage infection.
These data led us to hypothesize that OptS-synthesized nucleotides were actually inhibiting, rather than activating, the OptE effector. Upon phage infection, however, Acb2 sequesters the OptS product away from OptE, unleashing its growth inhibition effect.
March 31, 2025 at 10:00 PM
These data led us to hypothesize that OptS-synthesized nucleotides were actually inhibiting, rather than activating, the OptE effector. Upon phage infection, however, Acb2 sequesters the OptS product away from OptE, unleashing its growth inhibition effect.
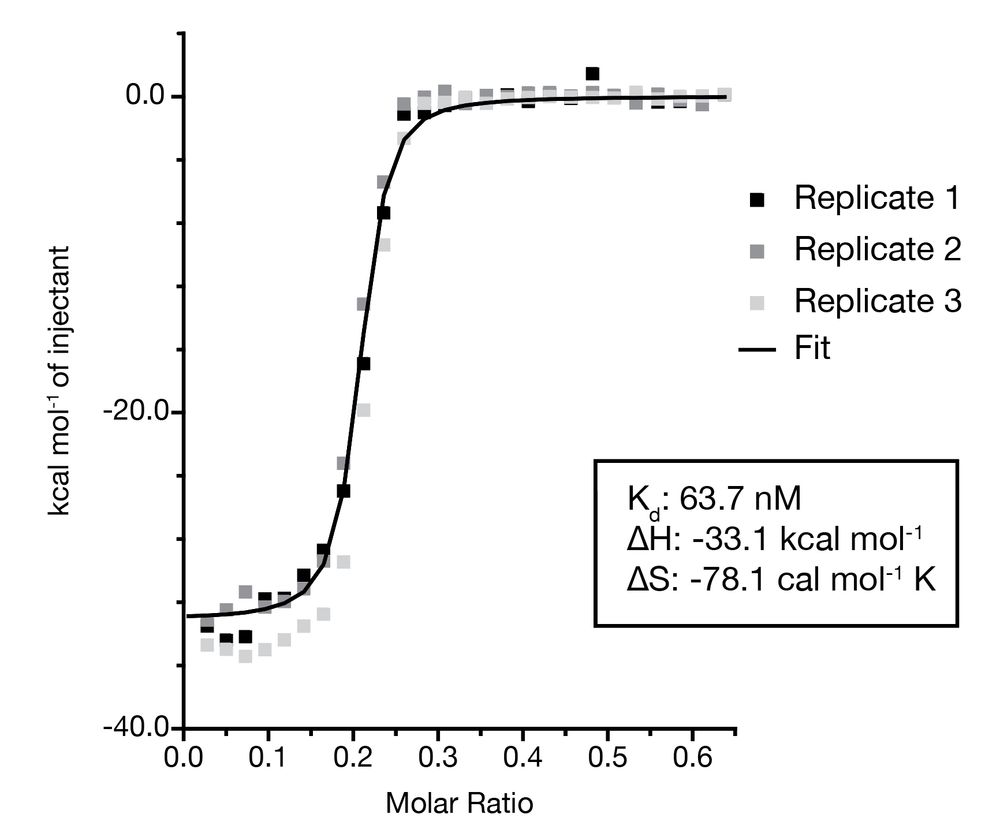
These findings left us puzzled: How does the Acb2 nucleotide sponge protein activate the Panoptes system? Based on its ability to bind diverse nucleotide products, we proposed that Acb2 also binds the product of OptS (2′,3′-c-di-AMP), which is exactly what we found.
March 31, 2025 at 10:00 PM
These findings left us puzzled: How does the Acb2 nucleotide sponge protein activate the Panoptes system? Based on its ability to bind diverse nucleotide products, we proposed that Acb2 also binds the product of OptS (2′,3′-c-di-AMP), which is exactly what we found.
Acb2 was recently discovered by the Bondy-Denomy and Chen Labs to be an anti-defense protein that acts as a nucleotide “sponge,” sequestering CBASS-derived cyclic oligonucleotide signaling molecules.
March 31, 2025 at 10:00 PM
Acb2 was recently discovered by the Bondy-Denomy and Chen Labs to be an anti-defense protein that acts as a nucleotide “sponge,” sequestering CBASS-derived cyclic oligonucleotide signaling molecules.
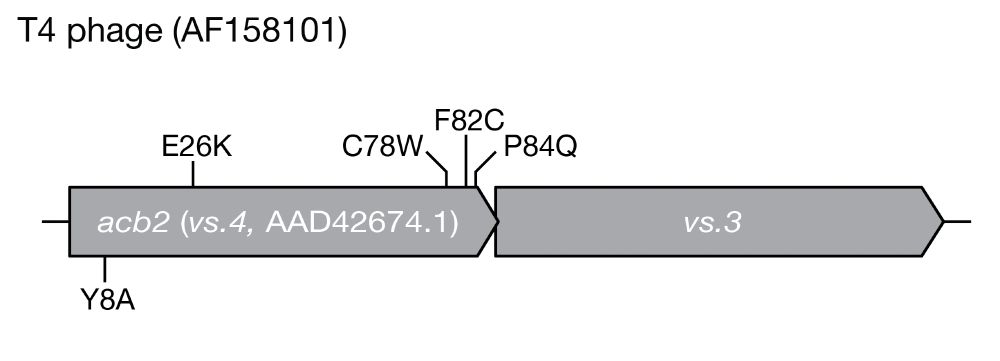
Intriguingly, the escaper phages we identified encoded loss-of-function mutations exclusively in the anti-CBASS 2 (acb2) gene. Consistent with this, we found that acb2 was necessary and sufficient to activate Panoptes defense.
March 31, 2025 at 10:00 PM
Intriguingly, the escaper phages we identified encoded loss-of-function mutations exclusively in the anti-CBASS 2 (acb2) gene. Consistent with this, we found that acb2 was necessary and sufficient to activate Panoptes defense.
We now knew that nucleotide signaling was a key component of the Panoptes system, but we were left wondering: how does the phage activate defense? To answer this question, we turned to phages that were able to escape Panoptes.
March 31, 2025 at 10:00 PM
We now knew that nucleotide signaling was a key component of the Panoptes system, but we were left wondering: how does the phage activate defense? To answer this question, we turned to phages that were able to escape Panoptes.
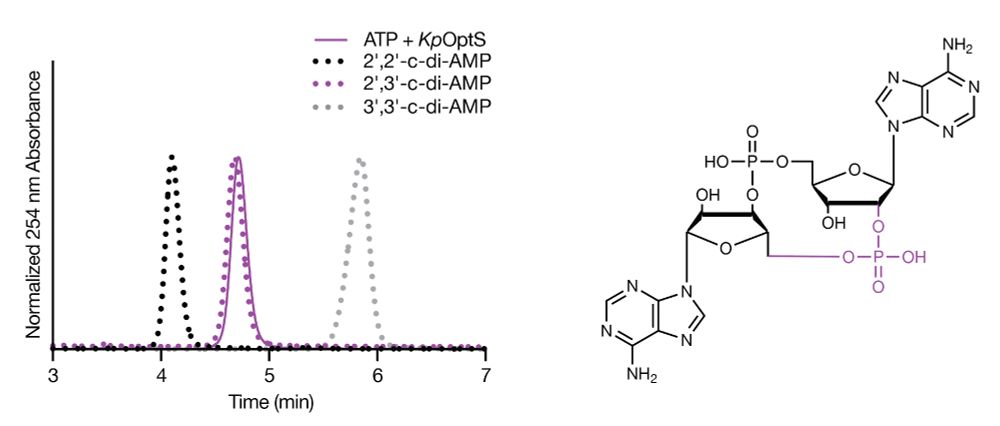
We tested the activity of OptS by incubating it with a range of NTPs and found that it used ATP as a substrate to specifically produce 2′,3′-c-di-AMP, an isomer of c-di-AMP. We used a series of phosphodiesterases to show that the linkage included a 3′–5′ and 2′–5′ bond.
March 31, 2025 at 10:00 PM
We tested the activity of OptS by incubating it with a range of NTPs and found that it used ATP as a substrate to specifically produce 2′,3′-c-di-AMP, an isomer of c-di-AMP. We used a series of phosphodiesterases to show that the linkage included a 3′–5′ and 2′–5′ bond.

