@NEJM study on @NuanceDragon DAX Copilot, an AI-powered documentation tool, found minimal impact on EHR efficiency & financial metrics❗️
💡AI tools need to be developed in comjuction with 🩺🥼🧑⚕️ not in silos
#AIinMedicine #LLMs @oncoalert.bsky.social
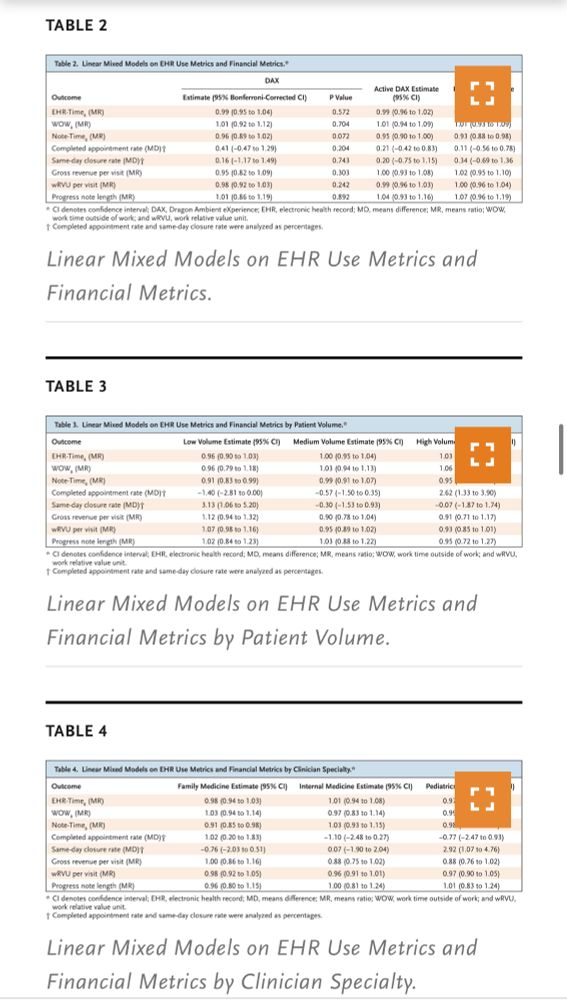
@NEJM study on @NuanceDragon DAX Copilot, an AI-powered documentation tool, found minimal impact on EHR efficiency & financial metrics❗️
💡AI tools need to be developed in comjuction with 🩺🥼🧑⚕️ not in silos
#AIinMedicine #LLMs @oncoalert.bsky.social
@FDA grants sacituzumab govitecan BTD for ES-SCLC progressing on platinum chemo, based on phase II TROPiCS-03 data:
🔹 ORR: 41.9%
🔹 Disease control: 83.7%
🔹 Clinical benefit: 48.8%
Next step: phase III trials. 🤞 this provides another option
#LCSM #SCLC


📈 Each month of 2024 has brought incredible advancements—remarkable strides in lung cancer treatment! 💉
✨ Here's another perspective on this impressive journey. Just Ah-mazing! @oncodaily.bsky.social @n8pennell.bsky.social @jackwestmd.bsky.social

📈 Each month of 2024 has brought incredible advancements—remarkable strides in lung cancer treatment! 💉
✨ Here's another perspective on this impressive journey. Just Ah-mazing! @oncodaily.bsky.social @n8pennell.bsky.social @jackwestmd.bsky.social
• 📊 ORR: 21.4% across 8 tumor types (incl. NSCLC, pancreatic & biliary)
• 🛡️ Safety: minimal myelosuppression; nausea & fatigue
• 🎯 MoA: Synthetic lethality in MTAP-deleted cells! @oncoalert.bsky.social
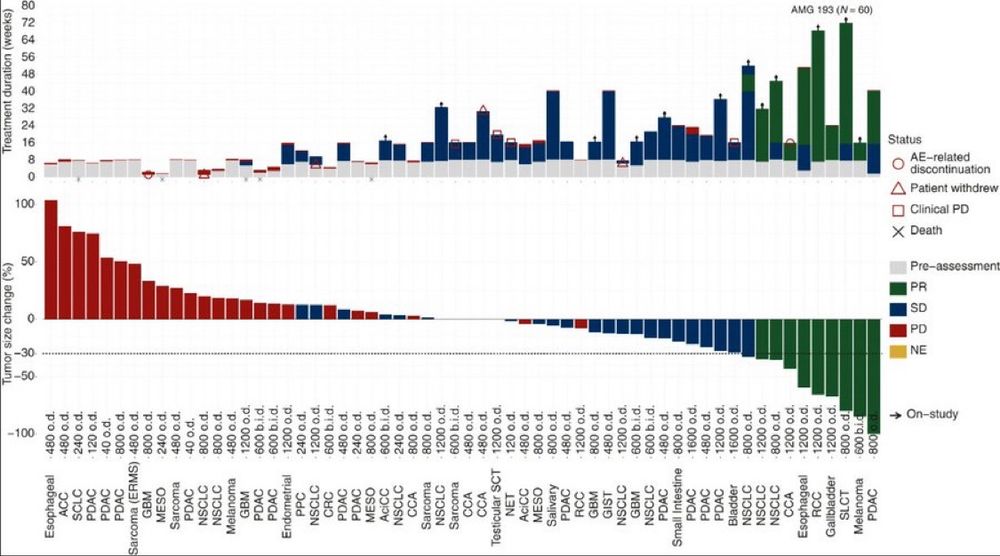
• 📊 ORR: 21.4% across 8 tumor types (incl. NSCLC, pancreatic & biliary)
• 🛡️ Safety: minimal myelosuppression; nausea & fatigue
• 🎯 MoA: Synthetic lethality in MTAP-deleted cells! @oncoalert.bsky.social
Durvalumab is now FDA-approved for limited-stage SCLC based on the ADRIATIC trial. Adding 2 years of durvalumab after chemoradiation improves both PFS and OS (HR 0.73).
This approval marks an important step forward for patients with limited-stage SCLC! 💪

Durvalumab is now FDA-approved for limited-stage SCLC based on the ADRIATIC trial. Adding 2 years of durvalumab after chemoradiation improves both PFS and OS (HR 0.73).
This approval marks an important step forward for patients with limited-stage SCLC! 💪
Excited to apply these insights to patient care! 🌟 #AIInMedicine #StudentforLife

Excited to apply these insights to patient care! 🌟 #AIInMedicine #StudentforLife
◾️ Median OOP cost of $98 (IQR: $43.8-$306.5)
◾️Biomarker testing total OOP cost of all services in 6 mths of diagnosis: $3560.2 compared to $1979.6 for those who did not! bit.ly/4hUTqaa

◾️ Median OOP cost of $98 (IQR: $43.8-$306.5)
◾️Biomarker testing total OOP cost of all services in 6 mths of diagnosis: $3560.2 compared to $1979.6 for those who did not! bit.ly/4hUTqaa
Based on HERIZON-BTC-01!
➡️ORR: 41.3%, mDOR=14.9 mths
➡️ Approval for IHC 3+, where TDX-d also has agnostic approval
➡️18% Gr3 TRAEs, diarrhea
#Oncosky @oncoalert.bsky.social @viveksubbiah.bsky.social


Based on HERIZON-BTC-01!
➡️ORR: 41.3%, mDOR=14.9 mths
➡️ Approval for IHC 3+, where TDX-d also has agnostic approval
➡️18% Gr3 TRAEs, diarrhea
#Oncosky @oncoalert.bsky.social @viveksubbiah.bsky.social
But navigating these options can be complex. Glad to partner with @OncLearnNetwork to create patient case-based education, bridging the gap between advancements and real-world application.
bit.ly/498ffPB

But navigating these options can be complex. Glad to partner with @OncLearnNetwork to create patient case-based education, bridging the gap between advancements and real-world application.
bit.ly/498ffPB

