
Adam Fountain
@adamfountain.bsky.social
PostDoc in the Ramakrishnan/Luisi labs at University of Cambridge.
Bedaquiline resistance in Mycobacterium tuberculosis #ENDTB
Bedaquiline resistance in Mycobacterium tuberculosis #ENDTB
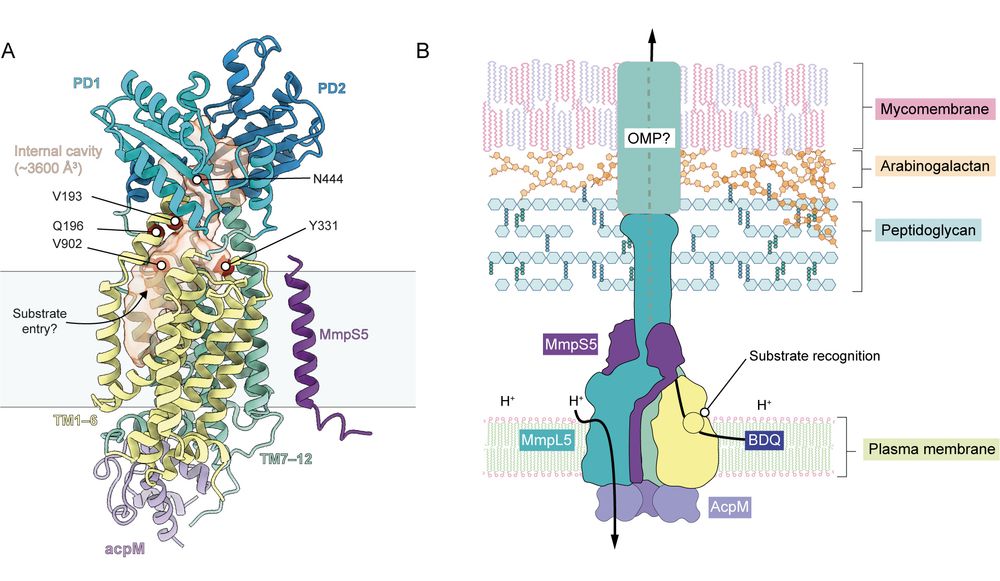
(6/8) Using three independent genetic approaches, we identified variants in MmpL5 that both increase and decrease bedaquiline efflux. These variants converge on a site the lower periplasmic cavity of MmpL5 that we propose is the binding site of bedaquiline.
June 26, 2025 at 10:30 AM
(6/8) Using three independent genetic approaches, we identified variants in MmpL5 that both increase and decrease bedaquiline efflux. These variants converge on a site the lower periplasmic cavity of MmpL5 that we propose is the binding site of bedaquiline.
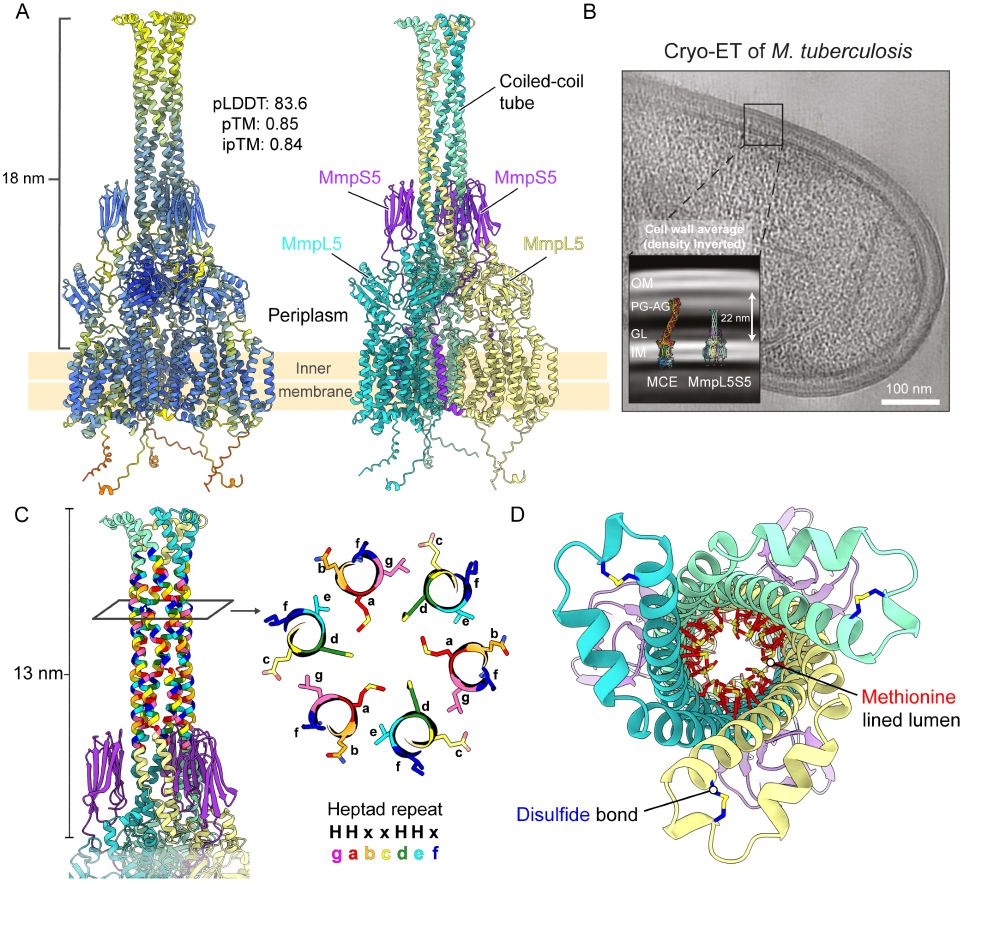
(4/8) MmpL5 proteins often have a puzzling 13 nm long coiled coil inserted in their periplasmic domain that is essential for efflux.
Using AlphaFold2, we found that this domain forms an unusual methionine-lined tube to transport substrates across the periplasm.
Using AlphaFold2, we found that this domain forms an unusual methionine-lined tube to transport substrates across the periplasm.
June 26, 2025 at 10:30 AM
(4/8) MmpL5 proteins often have a puzzling 13 nm long coiled coil inserted in their periplasmic domain that is essential for efflux.
Using AlphaFold2, we found that this domain forms an unusual methionine-lined tube to transport substrates across the periplasm.
Using AlphaFold2, we found that this domain forms an unusual methionine-lined tube to transport substrates across the periplasm.
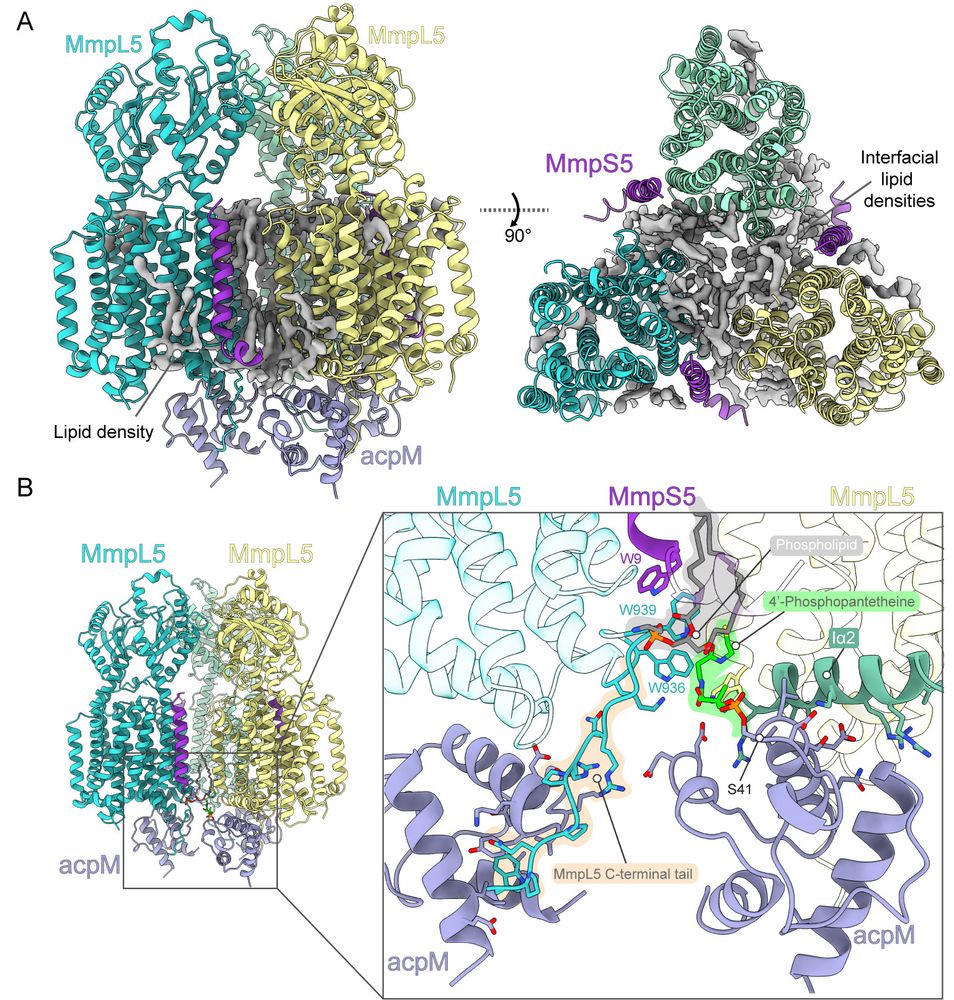
(3/8) Surprisingly, we found that the trimer interface is mediated by phospholipids (which might explain why purifying this had proved so challenging 😥). The acpM protein that co-purifies with MmpL5 sandwiches its Phosphopantetheine group into the subunit interface…
June 26, 2025 at 10:30 AM
(3/8) Surprisingly, we found that the trimer interface is mediated by phospholipids (which might explain why purifying this had proved so challenging 😥). The acpM protein that co-purifies with MmpL5 sandwiches its Phosphopantetheine group into the subunit interface…
(2/8) Our cryo-EM structure revealed that MmpS5L5 has a novel trimeric architecture distinct from the classic AcrAB-TolC pumps found in Gram-negative bacteria.
Mycobacteria always like to do things differently…
Mycobacteria always like to do things differently…

June 26, 2025 at 10:30 AM
(2/8) Our cryo-EM structure revealed that MmpS5L5 has a novel trimeric architecture distinct from the classic AcrAB-TolC pumps found in Gram-negative bacteria.
Mycobacteria always like to do things differently…
Mycobacteria always like to do things differently…

